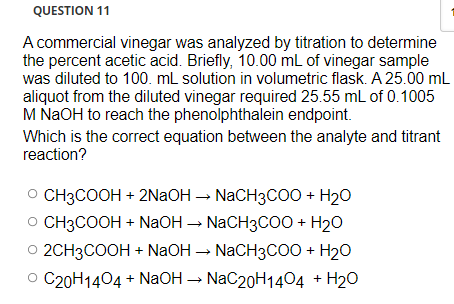
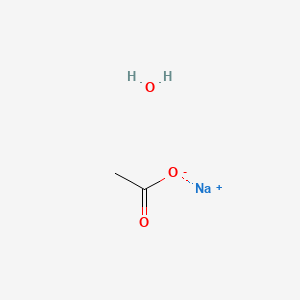
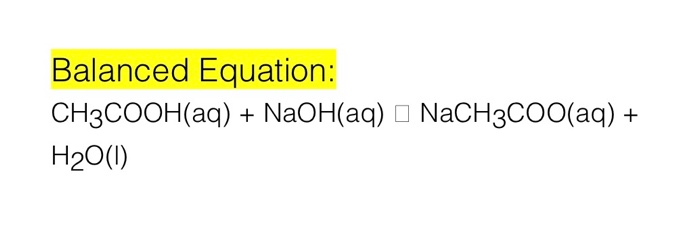
NaOH + X ----> NaCH3COO + H2O What is X in this reaction? A-NH4OH B-H3PO4 C-H2CO3 D-CH3COOH - brainly.com

In a reaction 5.3g of sodium carbonate reacted with 6g of ethanoic acid the products were 2.2g of carbon dioxide 0.9g water and 8.2g of sodium ethonoate show that these observations are

SOLVED: Sodium acetate (NaC2H3O2) is a basic salt. When sodium acetate is dissolved in water, it dissociates into its component ions. This reaction goes to completion, as indicated by the one-way arrow
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora

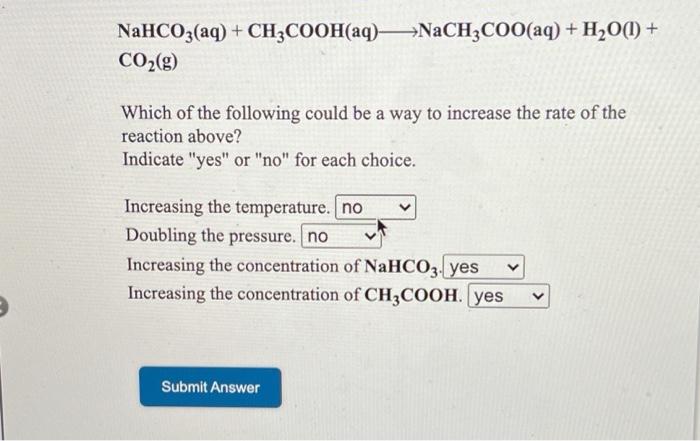
SOLVED: NaHCO3(aq) + CH3COOH(aq) â†' CO2(g) + NaCH3COO(aq) + H2O(l) Which of the following could be a way to increase the rate of the reaction above? Indicate "yes" or "no" for each

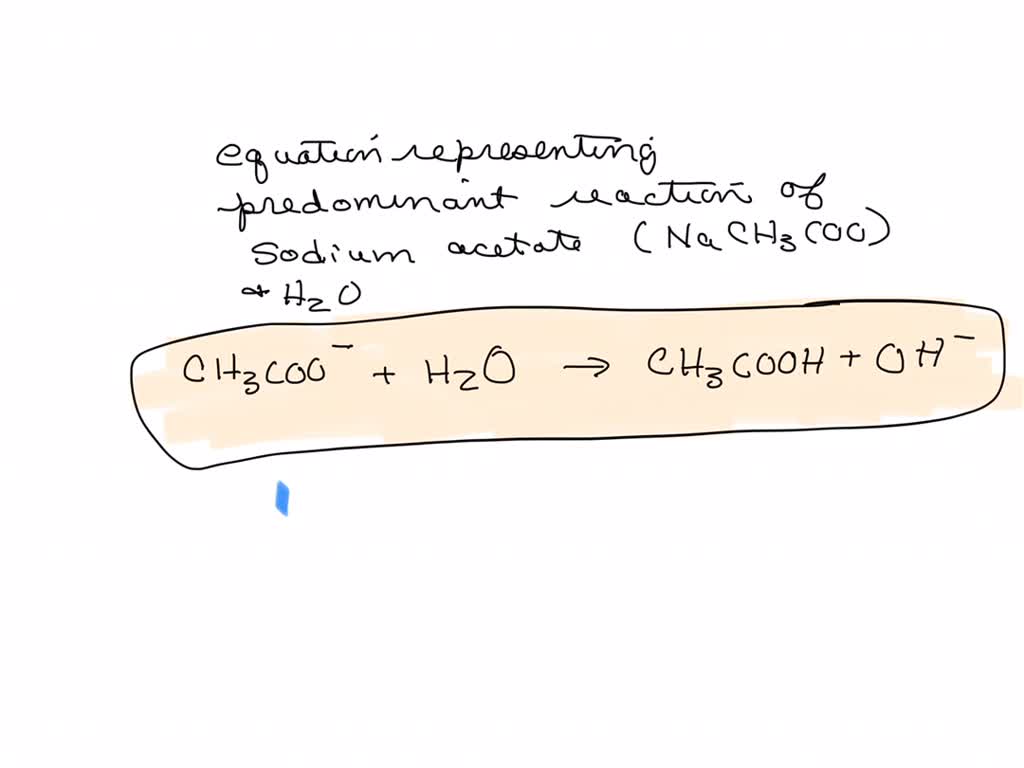
For sodium acetate solution in water, the given equilibrium reaction occur:CH 3 COO aq + H 2 O l hydrolysis ⇌ CH 3 COOH aq + OH aqWhich of the following describes

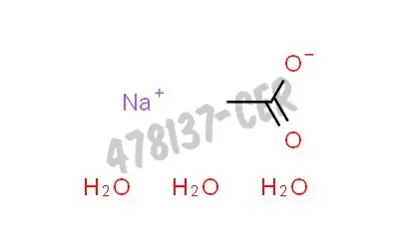
Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.