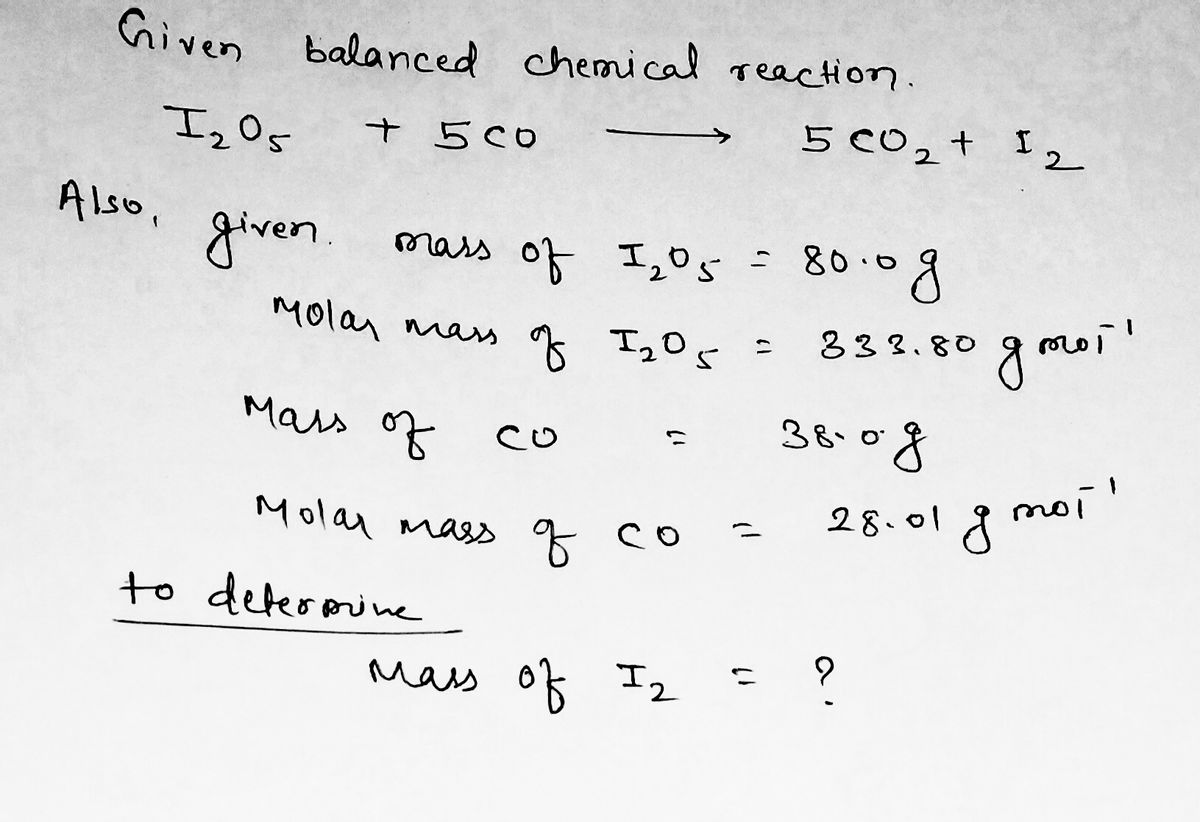I2O5-Mediated 1,5-Cyclization of Aryldiynes with H2O: A Way To Access 3-Acyl-1-indenone Derivatives - ScienceDirect

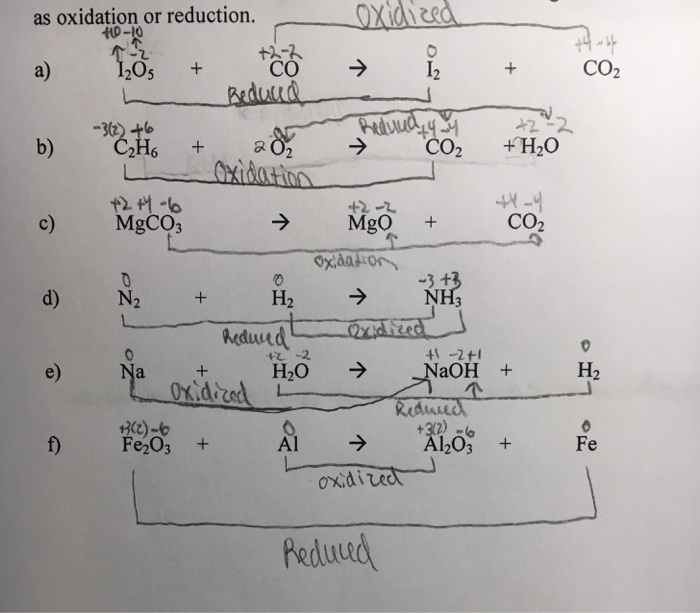
SOLVED: In each of the following balanced oxidation-reduction equations, identify those elements that undergo changes in oxidation number and indicate the magnitude of the change in each case. I2O5 (g) + 5
![I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.9b00765/asset/images/medium/jo-2019-007654_0002.gif)
I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry
![I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.9b00765/asset/images/acs.joc.9b00765.social.jpeg_v03)
I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry
![I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.9b00765/asset/images/medium/jo-2019-007654_0004.gif)
I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry
![SOLVED: What is the reagent required to accomplish the following transformation? OH OH PCC, ICl2, KMnO4, HIO4, RCO3H, I2O5/H2O [1] OsO4; [2] NaHSO3, H2O SOLVED: What is the reagent required to accomplish the following transformation? OH OH PCC, ICl2, KMnO4, HIO4, RCO3H, I2O5/H2O [1] OsO4; [2] NaHSO3, H2O](https://cdn.numerade.com/ask_images/507f98db453a458dbdd4504cf5b85177.jpg)