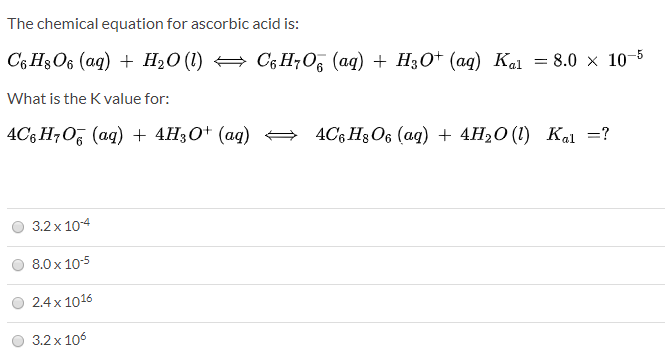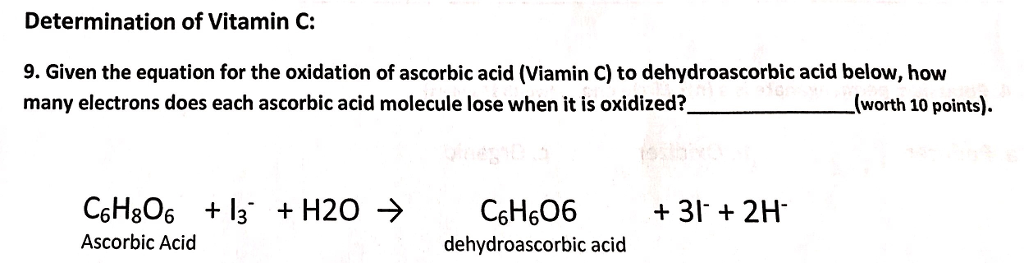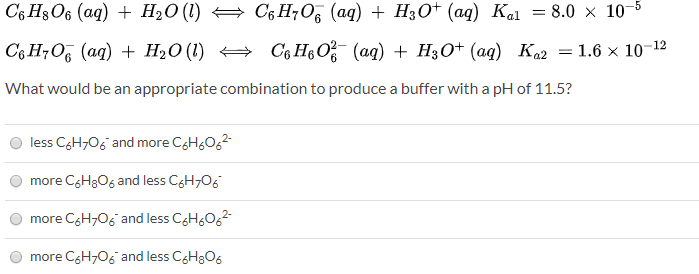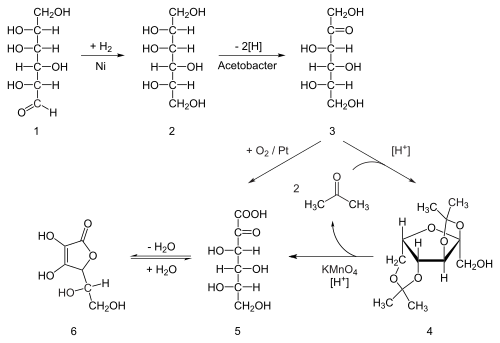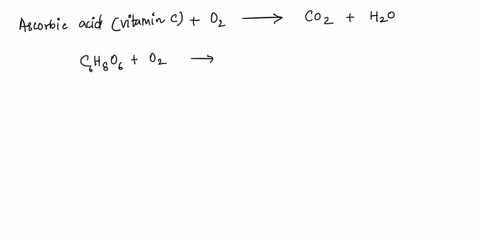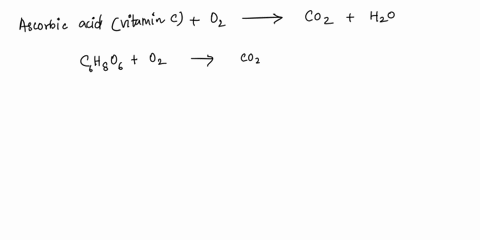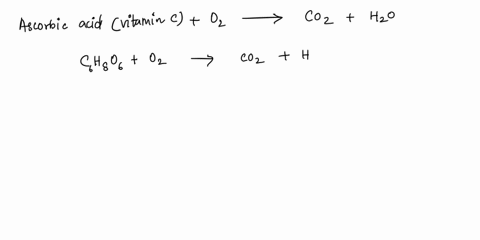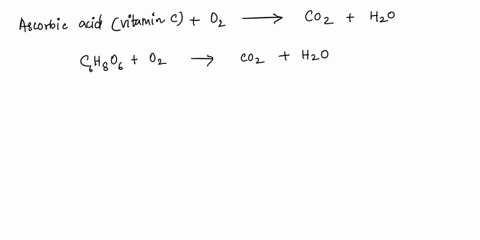
SOLVED: What is the balanced equation for the combustion of ascorbic acid ( C6H8O6, vitamin C) in oxygen to form CO2 and H2O? C6H8O6 + O2 -> CO2 + H2O

SOLVED: The chemical equation for ascorbic acid is: C6H8O6(aq) + H2O(l) â†' C6H7O6-(aq) + H3O+(aq) Change in G = 23.37 kJ What can be determined? The reaction is at equilibrium. The reaction
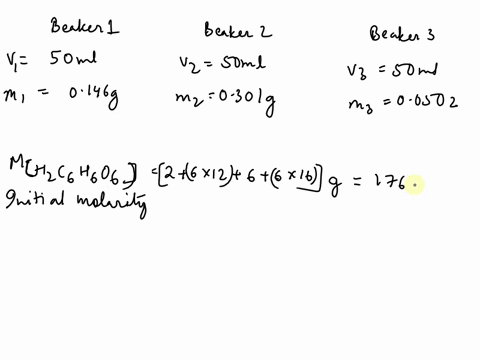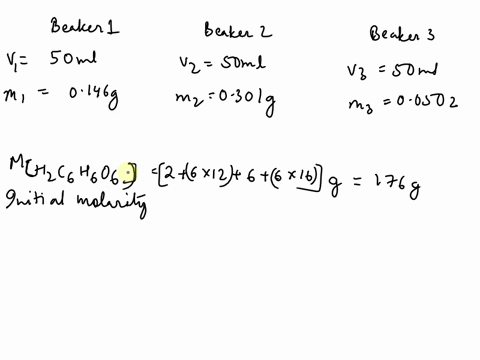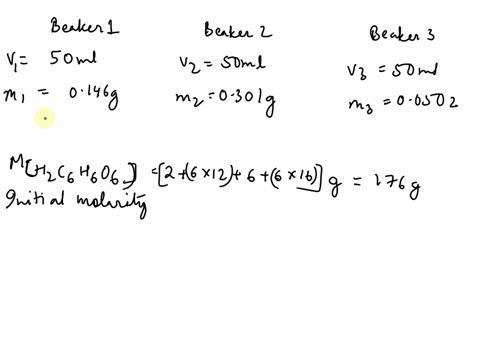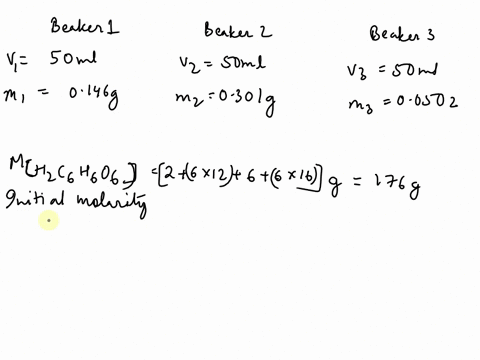
SOLVED: The chemical equation for ascorbic acid is: C6H8O6(aq) + H2O(l) â†' C6H7O6-(aq) + H3O+(aq) Change in G = 23.37 kJ What can be determined? The reaction is at equilibrium. The reaction
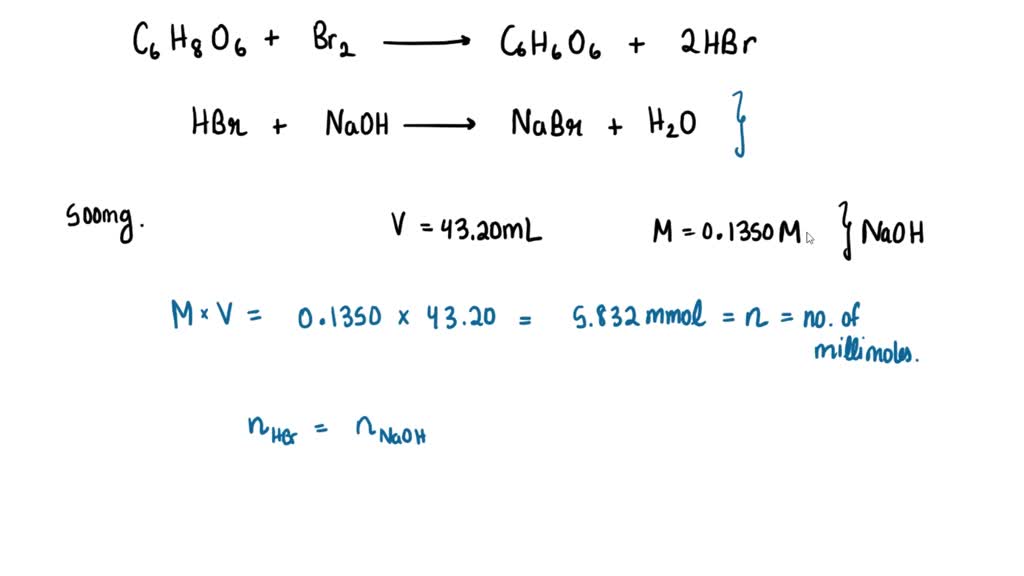
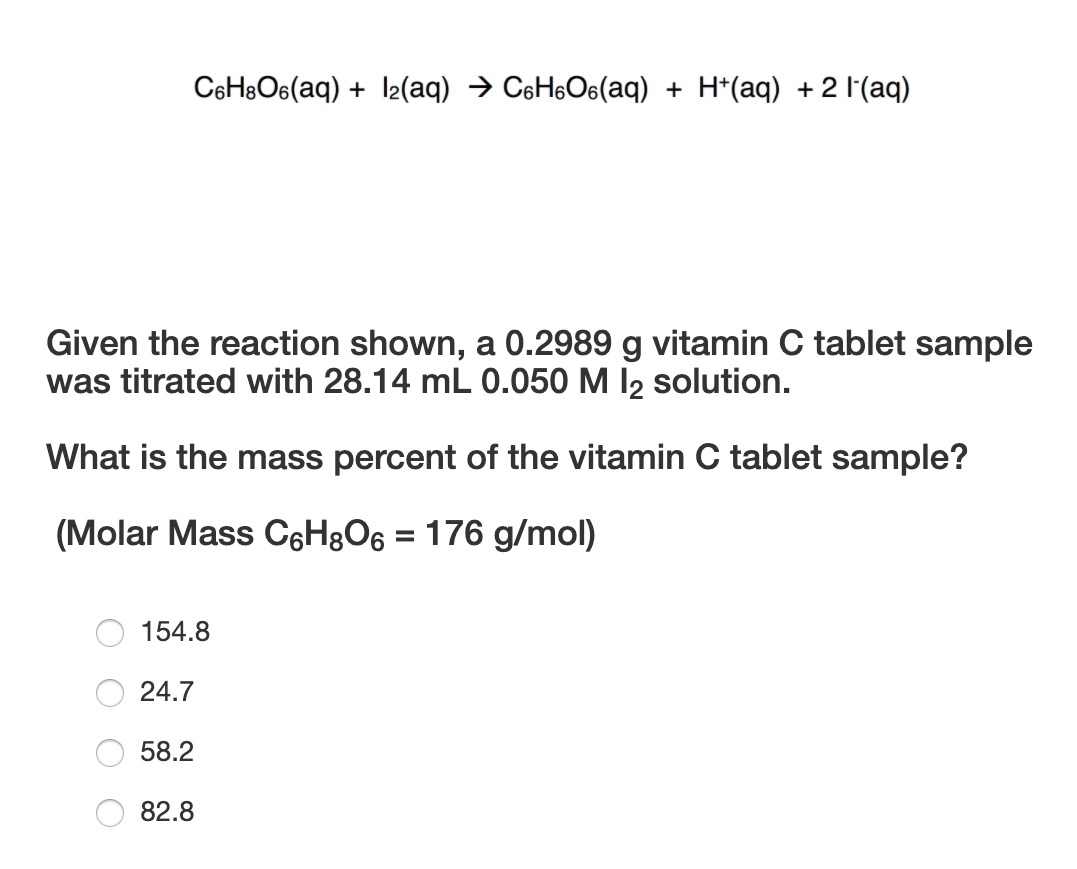
SOLVED: The amount of ascorbic acid (vitamin .C, C6H8O6) in tablets is determined by reaction with bromine and then titration of the hydrobromic acid with standard base: C6H8O6(a q)+Br2(a q) ⟶C6H6O6(a q)+2
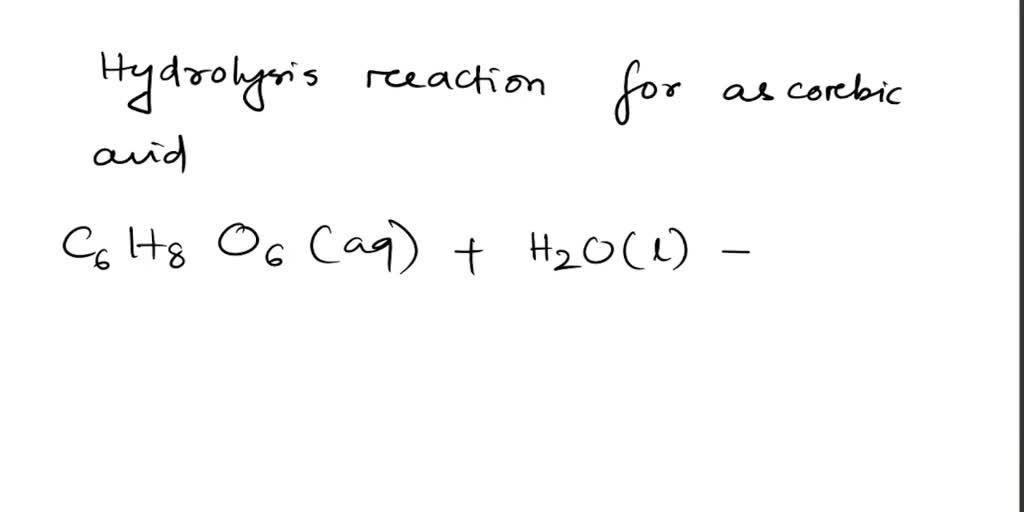
SOLVED: Absorbic Acid (C6H8O6) is a weak acid. a.) Write the hydrolysis reaction for ascorbic acid. b.) Write the acid-dissociation constant (Ka) expression for the reaction in part (a). c.) A 0.100

SOLVED: The chemical equation for ascorbic acid is: C6H8O6(aq) + H2O(l) â†' C6H7O6-(aq) + H3O+(aq) Change in G = 23.37 kJ What can be determined? The reaction is at equilibrium. The reaction

How the Multiple Antioxidant Properties of Ascorbic Acid Affect Lipid Oxidation in Oil-in-Water Emulsions | Journal of Agricultural and Food Chemistry

SOLVED: Again, consider an aqueous solution of ascorbic acid (Vitamin C, C6H8O6): (1) C6H8O6(aq) + H2O(l) = C6H6O6(aq) + H3O+(aq) Ka1 = 1.0x10^(-1) (2) C6H8O6-(aq) + H2O(l) = C6H6O6^2-(aq) + H3O+(aq) Ka2 =
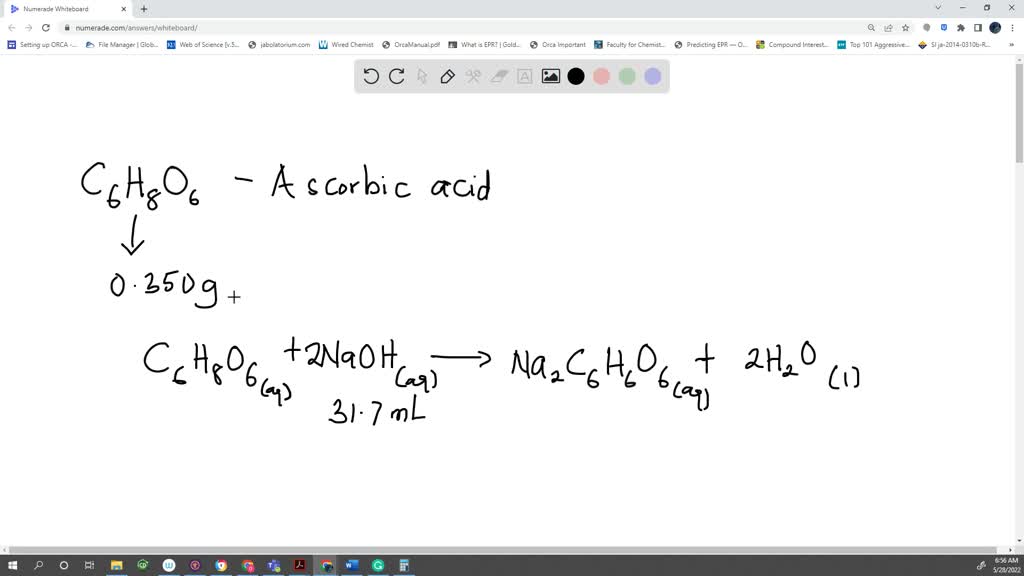
SOLVED: A sample of ascorbic acid, with the formula C6H8O6, is to be studied by titration. First, 0.350 grams of the acid are added to a flask. Sodium hydroxide solution is then
![SOLVED: Ascorbic acid (C6H8O6) is a diprotic acid. Its Ka values are listed below. What is the [C6H8O6-2] in a 0.15 M solution of ascorbic acid? C6H8O6 (aq) + H2O (l) ⇌ SOLVED: Ascorbic acid (C6H8O6) is a diprotic acid. Its Ka values are listed below. What is the [C6H8O6-2] in a 0.15 M solution of ascorbic acid? C6H8O6 (aq) + H2O (l) ⇌](https://cdn.numerade.com/ask_images/55e213a8c21947539aafd32122cc8bef.jpg)
SOLVED: Ascorbic acid (C6H8O6) is a diprotic acid. Its Ka values are listed below. What is the [C6H8O6-2] in a 0.15 M solution of ascorbic acid? C6H8O6 (aq) + H2O (l) ⇌
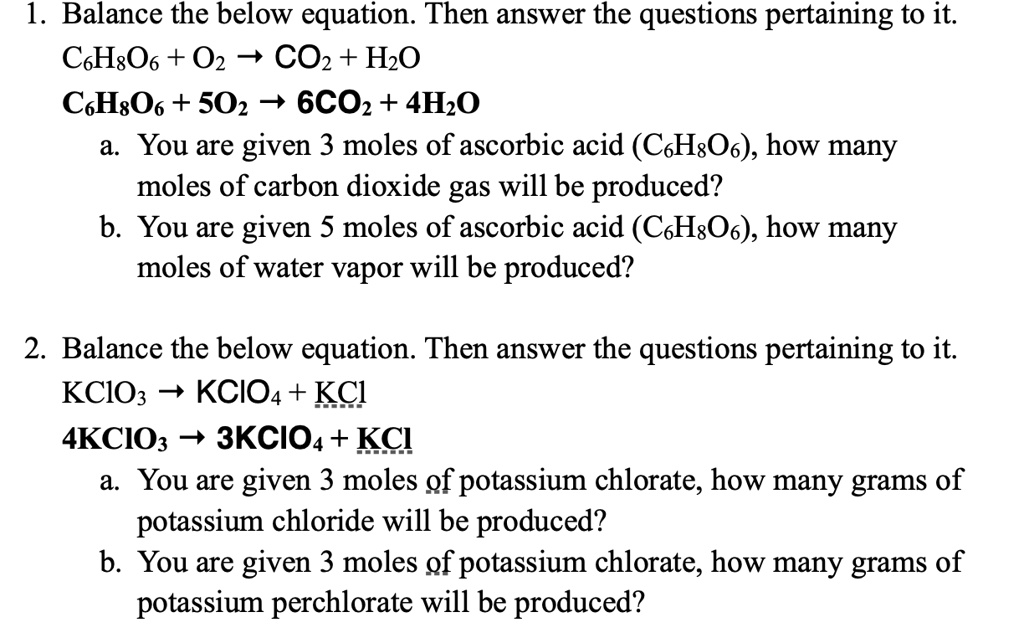
SOLVED: Balance the below equation. Then answer the questions pertaining to it. C6H8O6 + O2 -> 6CO2 + 4H2O CH2O + 5O2 -> 6CO2 + 4H2O a. You are given 3 moles

SOLVED: The chemical equation for ascorbic acid is: C6H8O6(aq) + H2O(l) â†' C6H7O6-(aq) + H3O+(aq) Change in G = 23.37 kJ What can be determined? The reaction is at equilibrium. The reaction