![SOLVED: In Al3+ + 6H2O â†' [Al(H2O)6]3+, what does H2O act as? Lewis base, Bronsted-Lowry base, Bronsted-Lowry acid, Lewis Acid 2. Correctedtext: What will happen if you remove a reactant from a SOLVED: In Al3+ + 6H2O â†' [Al(H2O)6]3+, what does H2O act as? Lewis base, Bronsted-Lowry base, Bronsted-Lowry acid, Lewis Acid 2. Correctedtext: What will happen if you remove a reactant from a](https://cdn.numerade.com/ask_previews/89fd73fd-55ff-4e1c-b8f2-49c556eb68c1_large.jpg)
SOLVED: In Al3+ + 6H2O â†' [Al(H2O)6]3+, what does H2O act as? Lewis base, Bronsted-Lowry base, Bronsted-Lowry acid, Lewis Acid 2. Correctedtext: What will happen if you remove a reactant from a
![Q.52 In the following [Al(H2O).)] + HCO3- [Al(H2O),OH)2 + HCO, (b) species behaving as Bronsted - Lowry acids are: Q.52 In the following [Al(H2O).)] + HCO3- [Al(H2O),OH)2 + HCO, (b) species behaving as Bronsted - Lowry acids are:](https://toppr-doubts-media.s3.amazonaws.com/images/2164712/a42abbba-24aa-49e2-9c74-16ac3879c732.jpg)
Q.52 In the following [Al(H2O).)] + HCO3- [Al(H2O),OH)2 + HCO, (b) species behaving as Bronsted - Lowry acids are:

a) Images of the Ga-In-Sn-Bi alloy-activated Al-H2O reaction process.... | Download Scientific Diagram
NaOH + PbO = x + H2O NaOH + SnO2 = y + H2O NaOH + H2O + Al = z + H2 Sum of number of atom present in one molecule

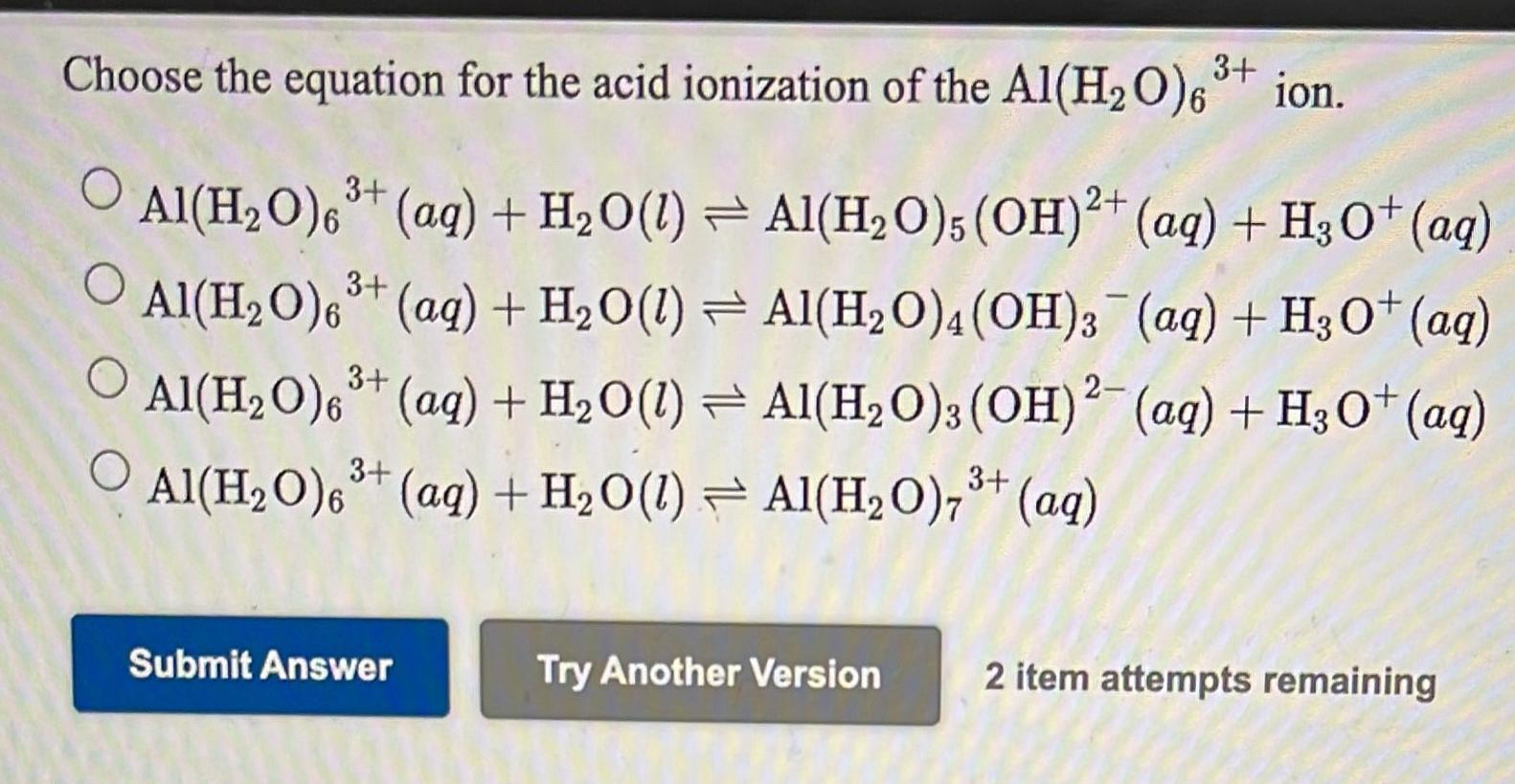
SOLVED: The hydrated Al3+ ion, Al(H2O)6^3+, is a weak acid in water. What are the products of its reaction with H2O? Al(H2O)6^3+(aq) + H2O(l) â†' Al( H2O)5(OH)2^-(aq) + H3O+(aq)
![SOLVED: In the reaction Al3+ + 6H2O → [Al(H2O)6]3+, what does H2O act as? Bronsted-Lowry acid Lewis acid Bronsted-Lowry Base Lewis base SOLVED: In the reaction Al3+ + 6H2O → [Al(H2O)6]3+, what does H2O act as? Bronsted-Lowry acid Lewis acid Bronsted-Lowry Base Lewis base](https://cdn.numerade.com/ask_previews/29288c5c-bf0a-455e-8d4b-2c5553f62277_large.jpg)




![What is the conjugate base of [Al (H2O)3 (OH)3]- ? Give the reason be - askIITians What is the conjugate base of [Al (H2O)3 (OH)3]- ? Give the reason be - askIITians](https://files.askiitians.com/cdn1/question-images/253949-screenshot_2019-08-19-13-20-17.png)