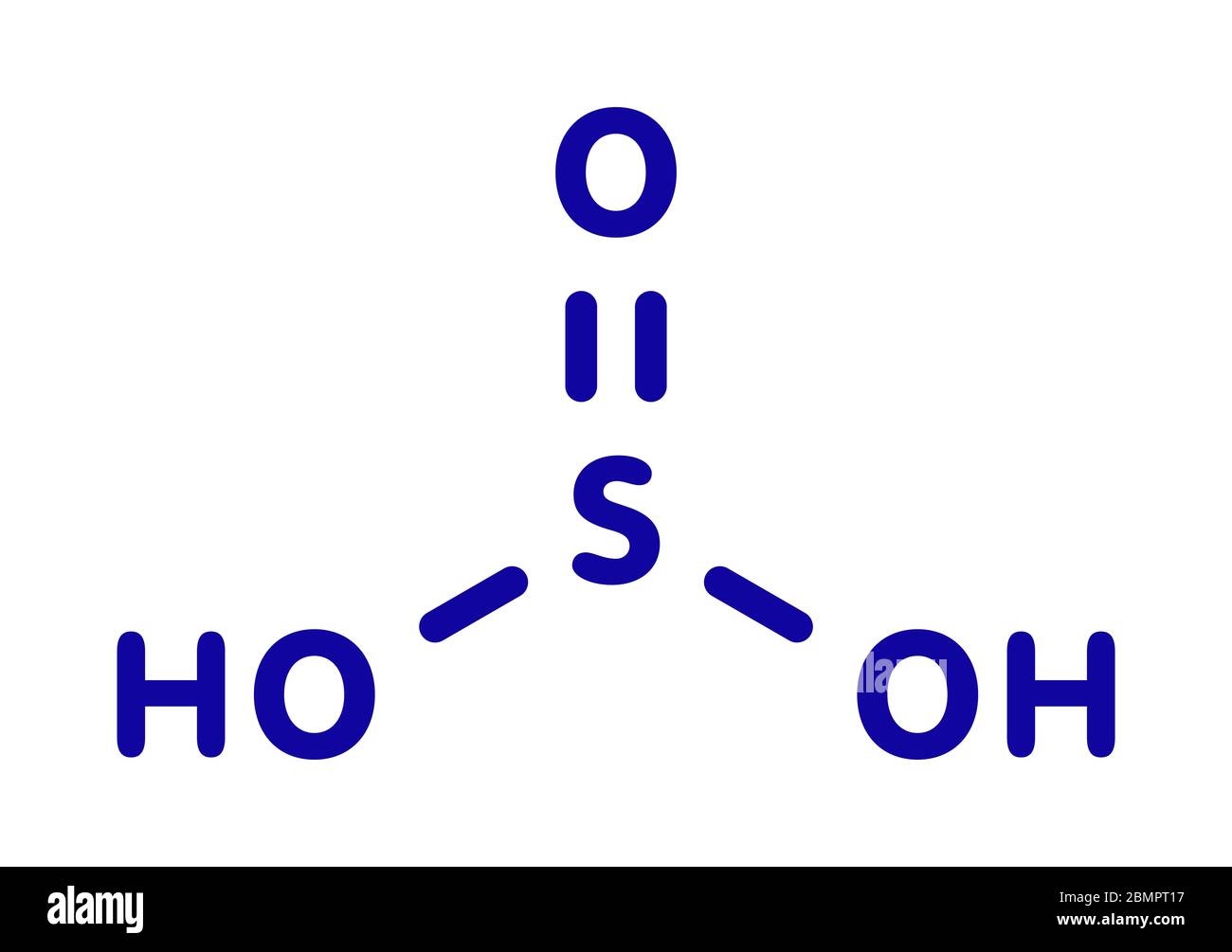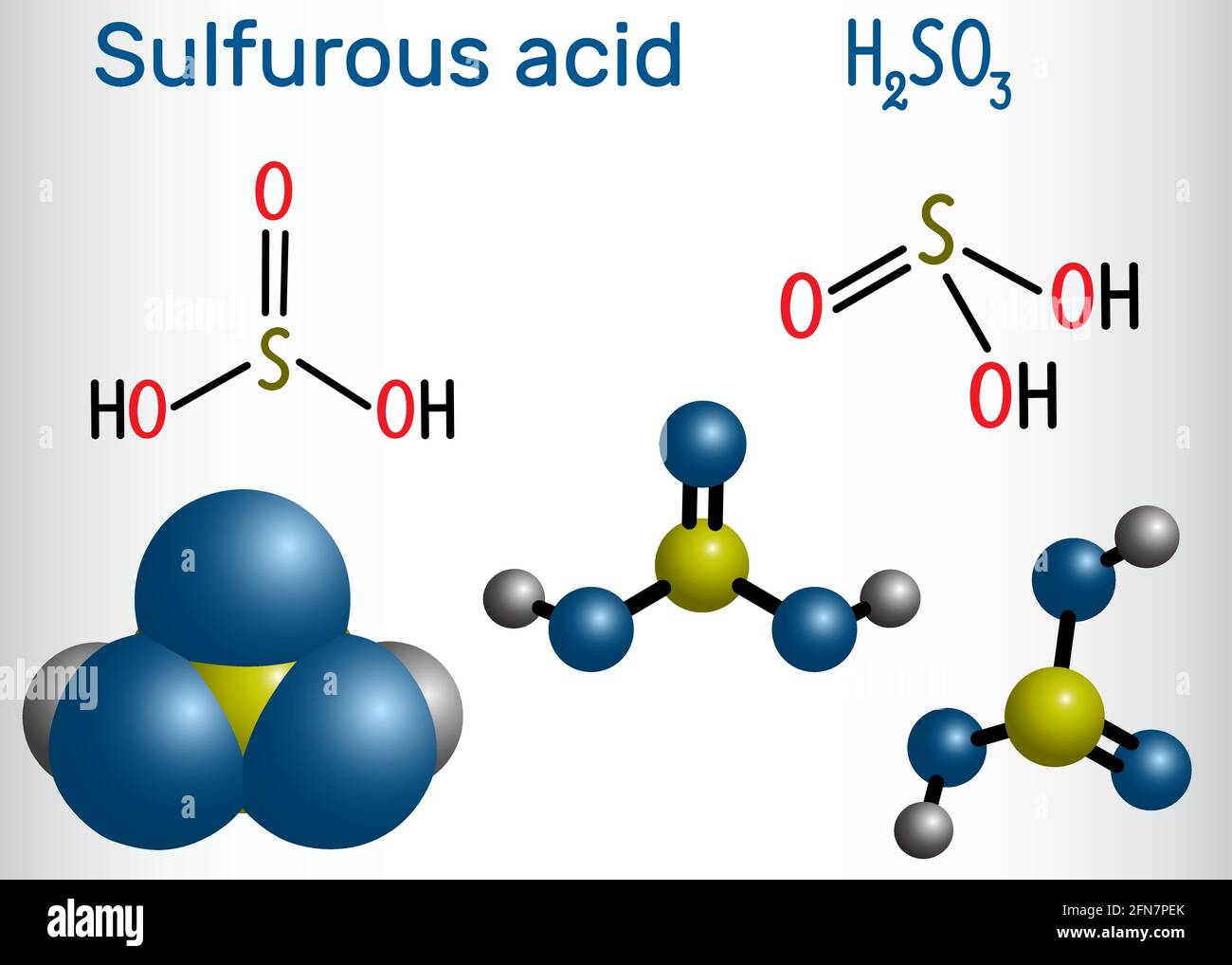
Molécule d'acide sulfurique (acide sulfurique, H2SO3). Formule chimique structurelle et modèle moléculaire. Illustration vectorielle Image Vectorielle Stock - Alamy

Figure 4 from Formic acid catalyzed hydrolysis of SO3 in the gas phase: a barrierless mechanism for sulfuric acid production of potential atmospheric importance. | Semantic Scholar

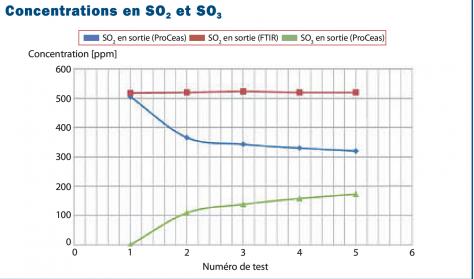
Kinetics of Sulfur Trioxide Reaction with Water Vapor to Form Atmospheric Sulfuric Acid | Journal of the American Chemical Society

Kinetics of Sulfur Trioxide Reaction with Water Vapor to Form Atmospheric Sulfuric Acid | Journal of the American Chemical Society

Kinetics of Sulfur Trioxide Reaction with Water Vapor to Form Atmospheric Sulfuric Acid | Journal of the American Chemical Society
if S + O2— > SO2 ; ( DH= 298.2 KJ ) SO2 +1/2O2——>SO3 ( DH= 98.7 KJ) SO3 + H2O——>H2SO4 (DH= 130.2 KJ) H2 + 1/2H2O—–> H2O ( DH= 287.3KJ) then
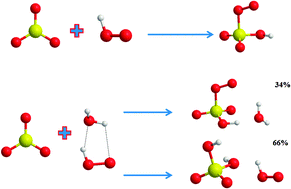
![Path sampling for atmospheric reactions: formic acid catalysed conversion of SO3 + H2O to H2SO4 [PeerJ] Path sampling for atmospheric reactions: formic acid catalysed conversion of SO3 + H2O to H2SO4 [PeerJ]](https://dfzljdn9uc3pi.cloudfront.net/2020/pchem-7/1/fig-1-2x.jpg)
Path sampling for atmospheric reactions: formic acid catalysed conversion of SO3 + H2O to H2SO4 [PeerJ]

Formic Acid Catalyzed Hydrolysis of SO3 in the Gas Phase: A Barrierless Mechanism for Sulfuric Acid Production of Potential Atmospheric Importance | Journal of the American Chemical Society

Kinetics of Sulfur Trioxide Reaction with Water Vapor to Form Atmospheric Sulfuric Acid | Journal of the American Chemical Society

Complexe souffre-trioxyde-pyridine, technique, SO3 actif à 48-50 %, Thermo Scientific Chemicals | Fisher Scientific