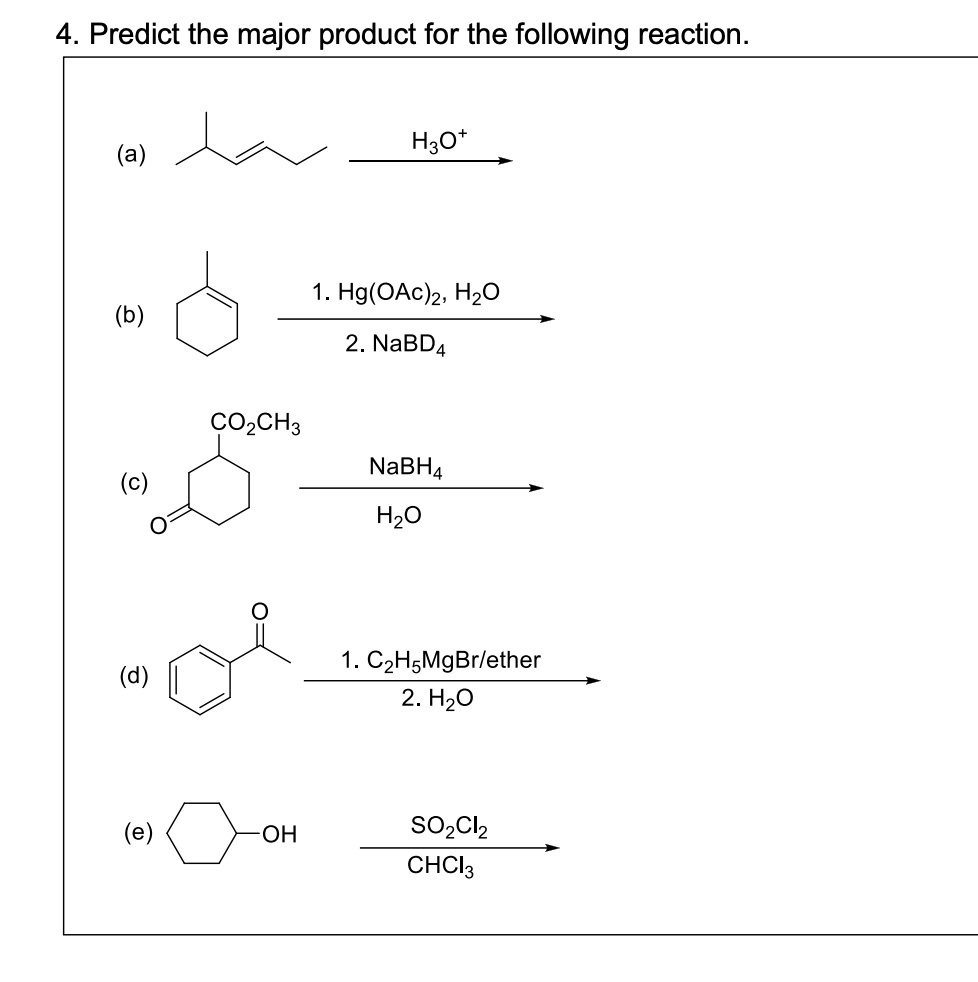
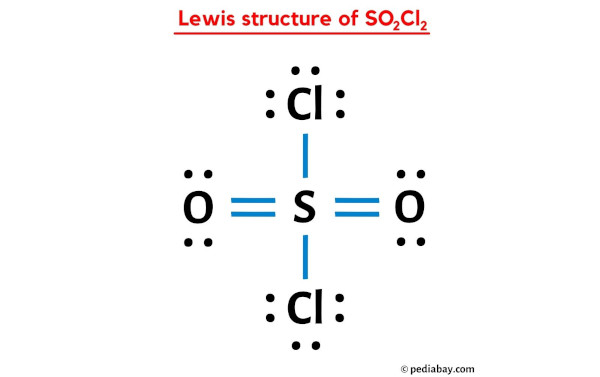
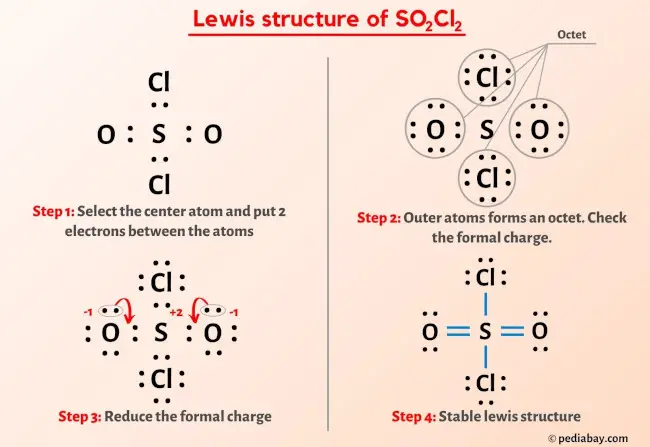
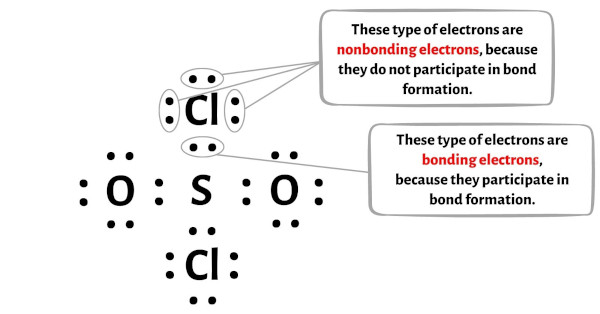
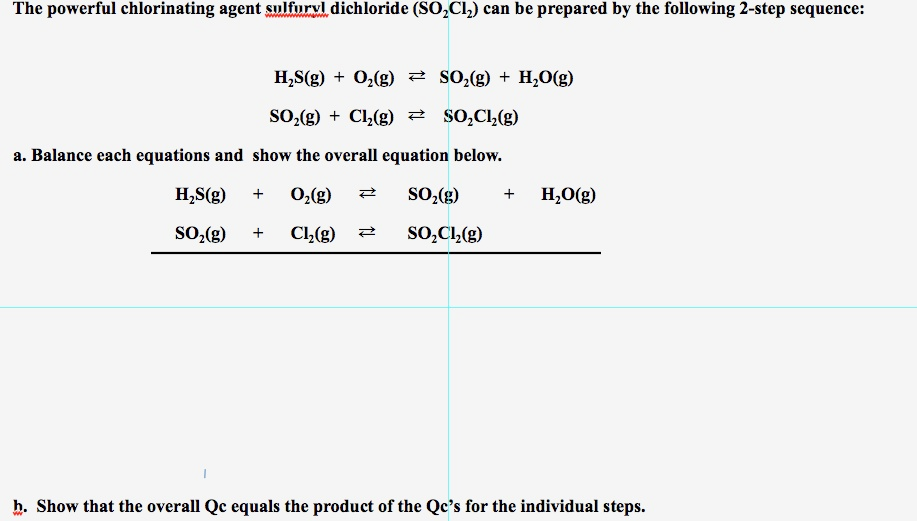
19. Complete the following equations: i) PA+ SO2Cl2 -- ii) XeF6 + H2O --------- iii) Cu + HNO3 (Conc.) ---

1 mole of SO2Cl2 is dissolved in water and Ca(OH)2 is added to neutralise acidic solution. The number of moles of Ca(OH)2 required is ______.
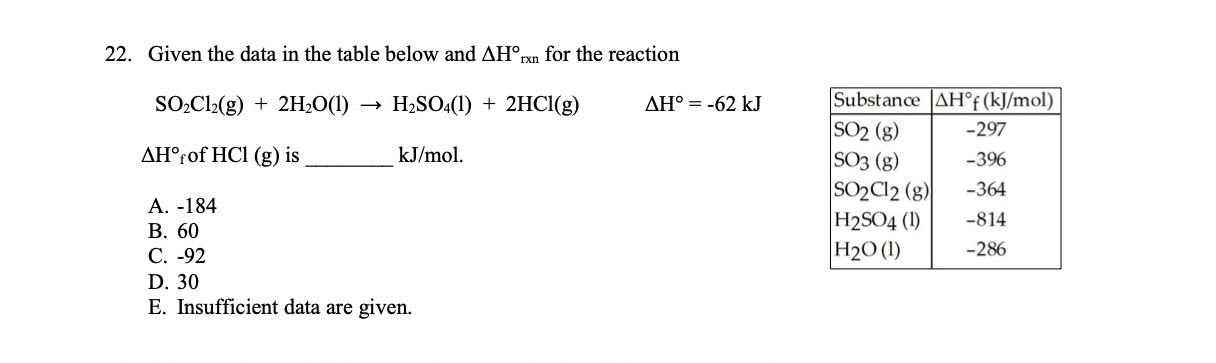
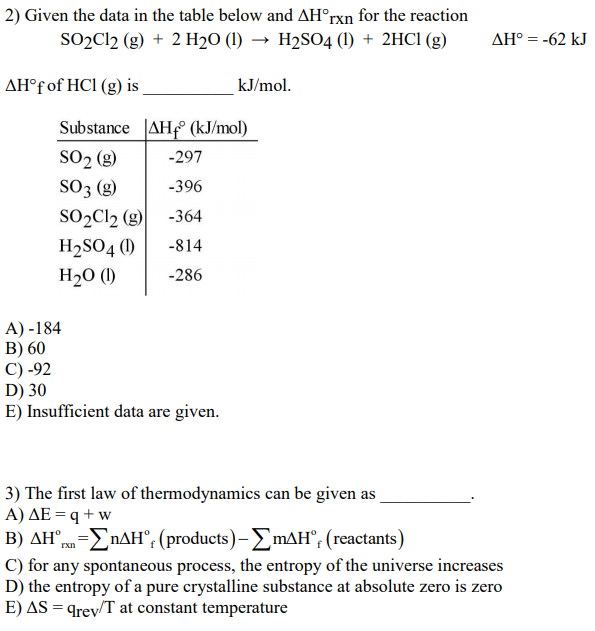
SOLVED: 22. Given the data in the table below and ΔH^∘rxn for the reaction SO2Cl2( g)+2 H2O(l) →H2SO4(l)+2 HCl(g) ΔH^∘=-62 kJ ΔH^∘f of HCl(g) is kJ / mol. A. -184 B. 60
SO2Cl2 hydrolyse as SO2Cl2 + 2H2O → H2SO4 + 2HCI For complete neutralization of product 16 mole of NaOH are required then - Sarthaks eConnect | Largest Online Education Community
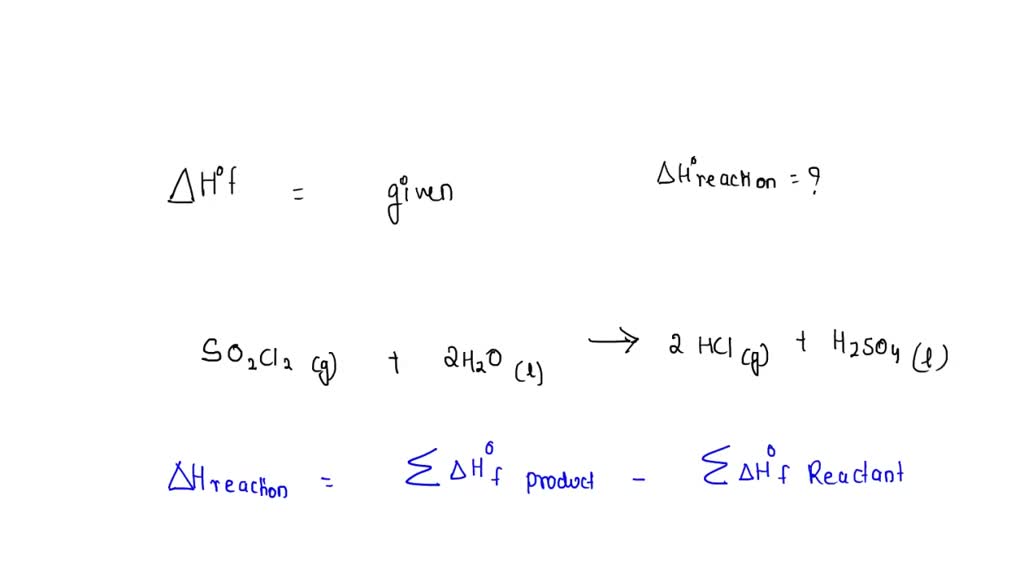
SOLVED: Use the ΔH°f information provided to calculate ΔH°rxn for the following: ΔH°f (kJ/mol) SO2Cl2(g) + 2 H2O(l) â†' 2 HCl(g) + H2SO4(l) ΔH°rxn = ? SO2Cl2(g) -364 H2O(l) -286 HCl(g) -92

19. Complete the following equations: i) PA+ SO2Cl2 -- ii) XeF6 + H2O --------- iii) Cu + HNO3 (Conc.) ---
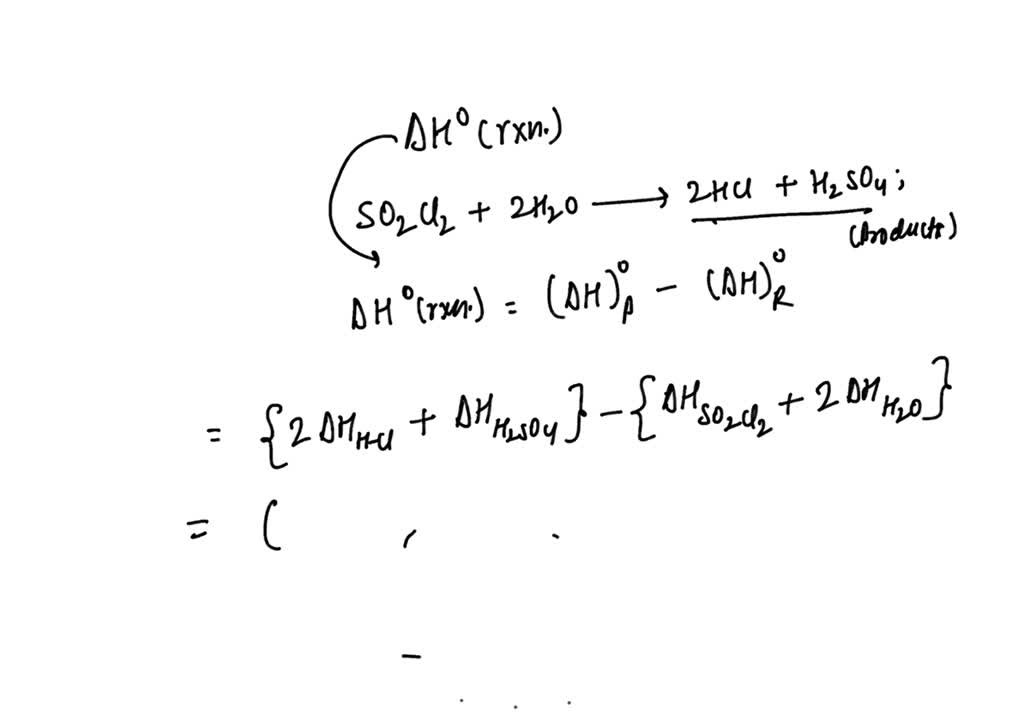
SOLVED: Calculate the ΔH°rxn for the following reaction: SO2Cl2 + 2H2O â†' 2HCl + H2SO4, using the enthalpy of formations. ΔH°f Given: SO2Cl2 = -364 kJ/mol H2O = -286 kJ/mol HCl = -

Sulphuryl chloride SO2Cl2, reacts with H2O to give mixture of H2SO4 and HCl. Aqueous solution of 1mole of SO2Cl2 will be neutralized by:a)3 moles of NaOHb)2 moles of Ca(OH)2c)Bothd)None of these Correct

SOLVED: Consider the reaction SO2Cl2 (g) + 2 H2O (l) + 2 HCl (g) â†' H2SO4 (l). Using the information in the table below, calculate the standard enthalpy of reaction ΔH°rxn for

53. Sul CU (3) CIO Sulphuryl chloride SO.CI, reacts with H,O to (4) MnO give mixture of H.SO, and HCL. Aq. solution 1 of 1 mol so.Cl, will be neutralised by: (1)