Calculated physical parameters such as energy (E) as eV, total dipole... | Download Scientific Diagram

Kinetic Study of Ni and NiO Reactions Pertinent to the Earth's Upper Atmosphere | The Journal of Physical Chemistry A

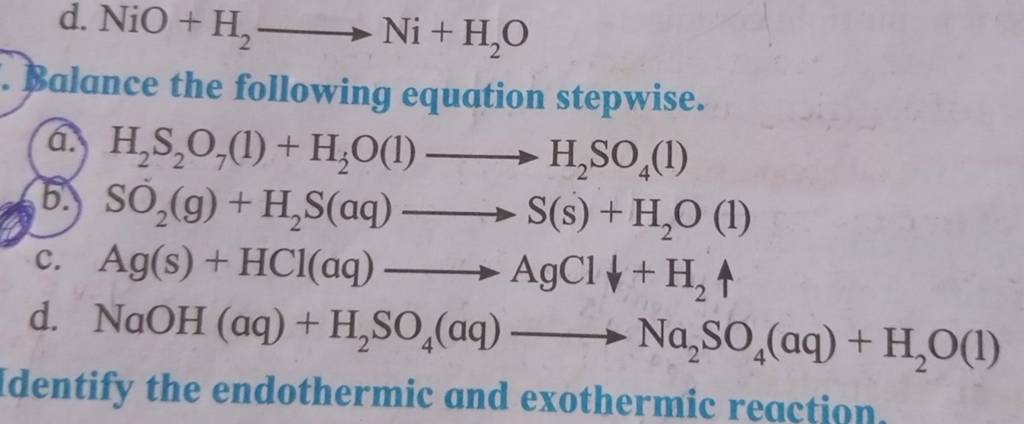
Identify from the following reactions the reactants that undergo oxidation and reduction.NiO+H2⟶Ni+H2O
![SOLVED: Which is the correct equilibrium constant expression for the following reaction? NiO(s) + H2(g) Ni(aq) + H2O(g) a) Kc = [H2]/[H2O] b) Kc = [Ni][H2O]/[Ni2O3] [H2] c) Kc = [Ni] [H2O]/[H2] SOLVED: Which is the correct equilibrium constant expression for the following reaction? NiO(s) + H2(g) Ni(aq) + H2O(g) a) Kc = [H2]/[H2O] b) Kc = [Ni][H2O]/[Ni2O3] [H2] c) Kc = [Ni] [H2O]/[H2]](https://cdn.numerade.com/project-universal/previews/f52234cd-9af7-477c-b7a1-a1075ed3eb39.gif)
SOLVED: Which is the correct equilibrium constant expression for the following reaction? NiO(s) + H2(g) Ni(aq) + H2O(g) a) Kc = [H2]/[H2O] b) Kc = [Ni][H2O]/[Ni2O3] [H2] c) Kc = [Ni] [H2O]/[H2]

Kinetic Study of Ni and NiO Reactions Pertinent to the Earth's Upper Atmosphere | The Journal of Physical Chemistry A

Wet etch rate of NiO in 1:4 HNO3:H2O as a function of solution temperature. | Download Scientific Diagram

Construction of NiO/H2Ti3O7 nanotube composite and its photocatalytic conversion feature for ethyl mercaptan | Applied Physics A

Ellingham diagram for Cu–CuO, NI–NiO, H2–H2O, CHP–H2O, and H–H2O [61,... | Download Scientific Diagram

IJMS | Free Full-Text | One Step Synthesis of NiO Nanoparticles via Solid-State Thermal Decomposition at Low-Temperature of Novel Aqua(2,9-dimethyl-1,10-phenanthroline)NiCl2 Complex

Single-Orientation Nanoporous NiO Films: Spontaneous Evolution from Dense Low-Crystalline Ni(OH)x Films | Crystal Growth & Design
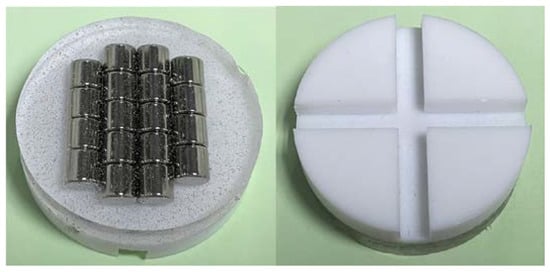
Coatings | Free Full-Text | Tribo-Catalytic Conversions of H2O and CO2 by NiO Particles in Reactors with Plastic and Metallic Coatings
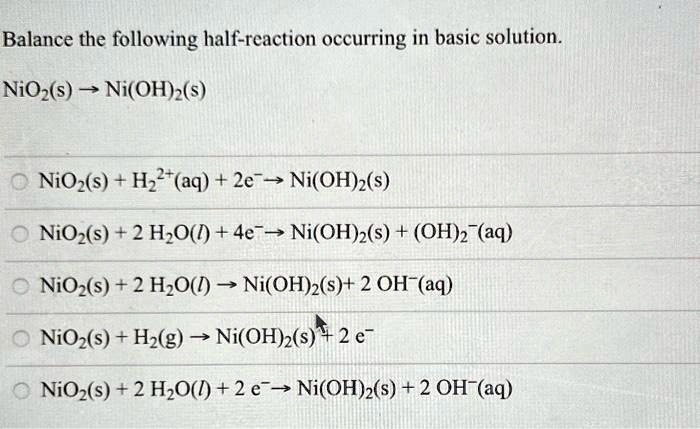
SOLVED: Balance the following half-reaction occurring in basic solution. NiO(s) - Ni(OH)2(s) NiO(s) + H2O(l) + 2e- -> Ni(OH)2(s) NiO(s) + 2H2O(l) + 4e- -> Ni(OH)2(s) + 4OH-(aq) O + NiO(s) +

Sm doped NiO catalysts with excellent H2O resistance for N2O decomposition under simulated nitric acid plant exhaust condition - ScienceDirect
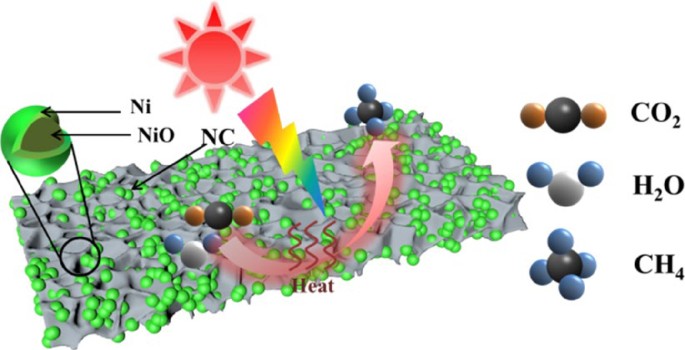
NiO@Ni nanoparticles embedded in N-doped carbon for efficient photothermal CO2 methanation coupled with H2O splitting | Nano Research