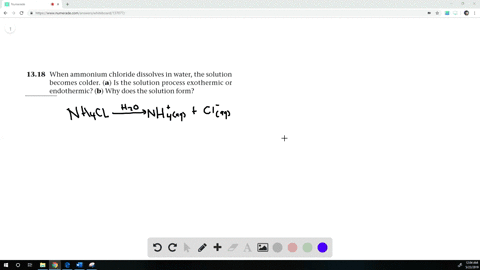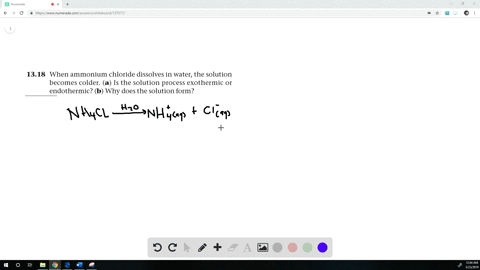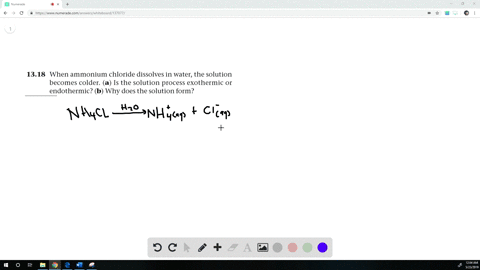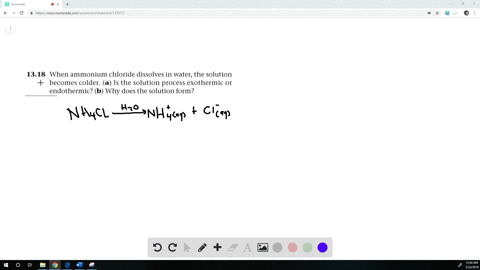
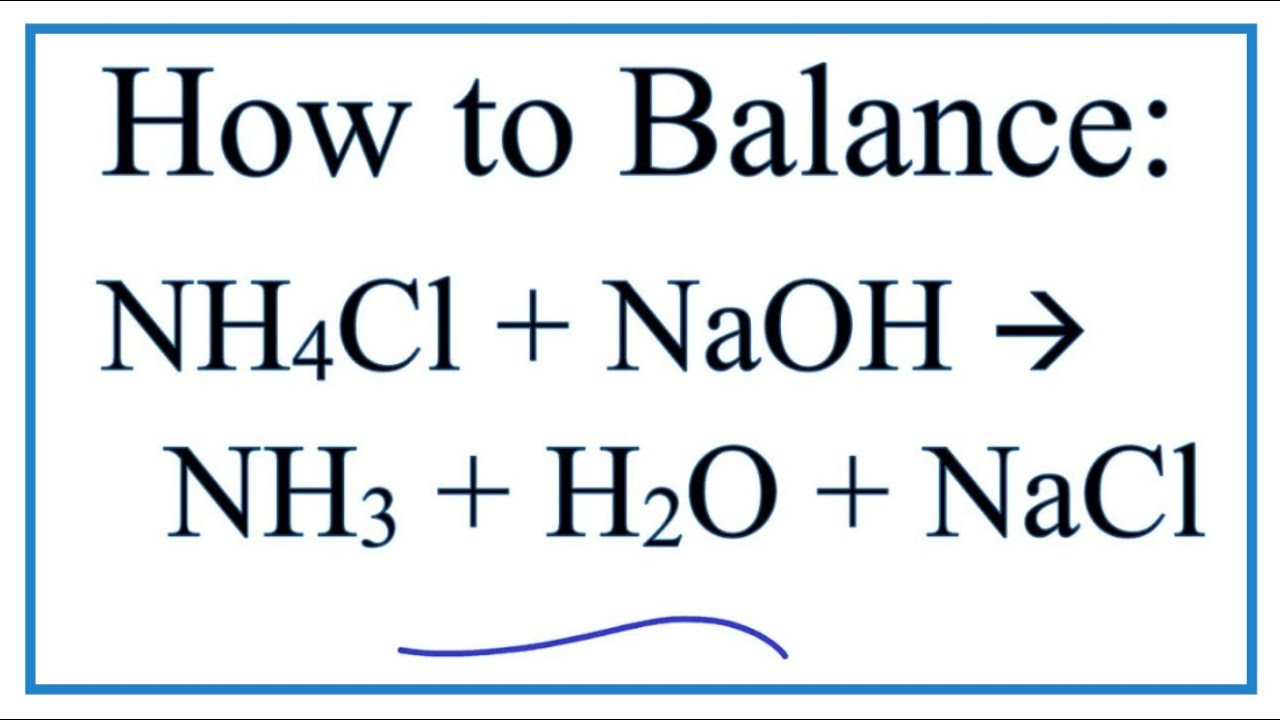
Equilibrium phase diagram for ammonium chloride-water solution. [25] We... | Download Scientific Diagram
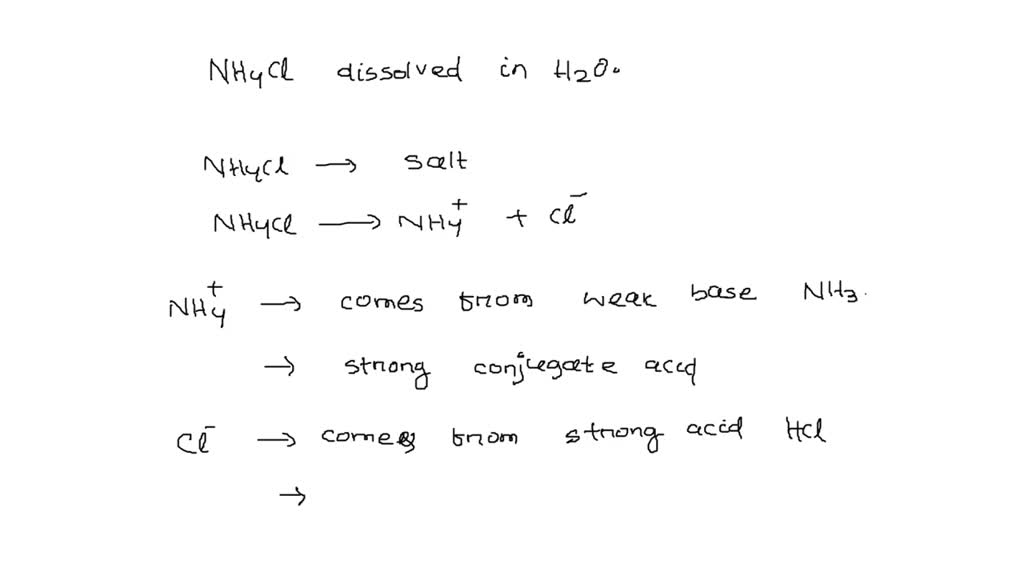
SOLVED: Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium chloride is dissolved in water. (Use H3O+ instead of H+.) NH4+(aq) + H2O(l) = NH3(aq) +
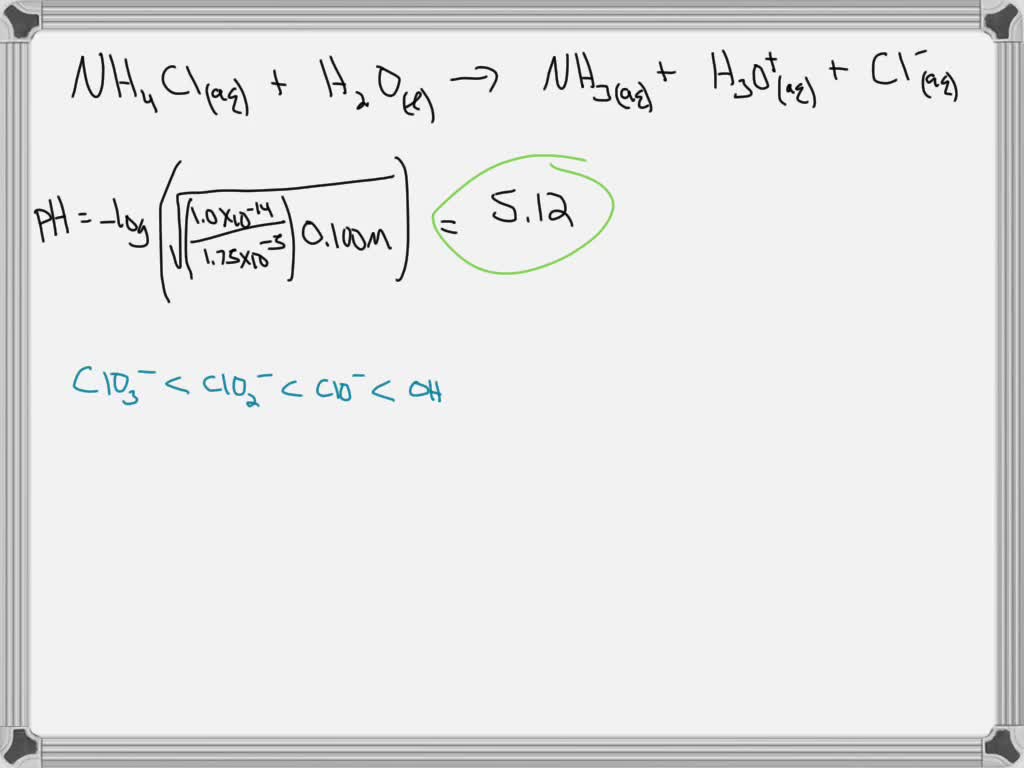
SOLVED: 1a. Write the reaction of ammonium chloride, NH4Cl, with water. - Calculate the pH of a 0.100 M solution of NH4Cl. The Kb of ammonia, NH3, is 1.75x10^-5. pH = 1b.

For a ammonium chloride solution in water, the given equilibrium reaction occur:NH 4+ aq + H 2 O l hydrolysis ⇌ NH 4 OH aq + H +aqWhich of the following describes
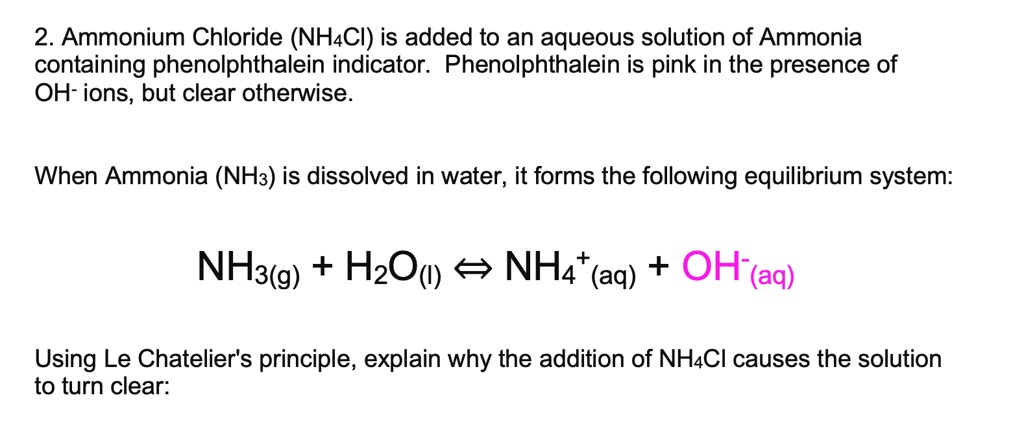
SOLVED: Ammonium Chloride (NH4Cl) is added to an aqueous solution of Ammonia containing phenolphthalein indicator. Phenolphthalein is pink in the presence of OH- ions, but clear otherwise. When Ammonia (NH3) is dissolved

Transmission IR spectra of 9:100 ammonium chloride/water solutions of... | Download Scientific Diagram