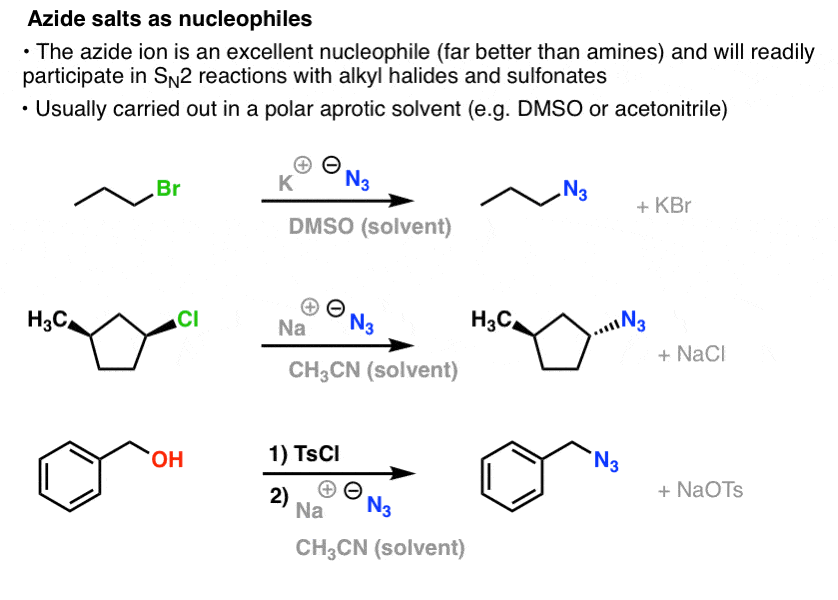
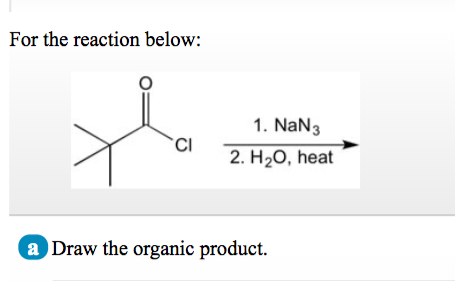
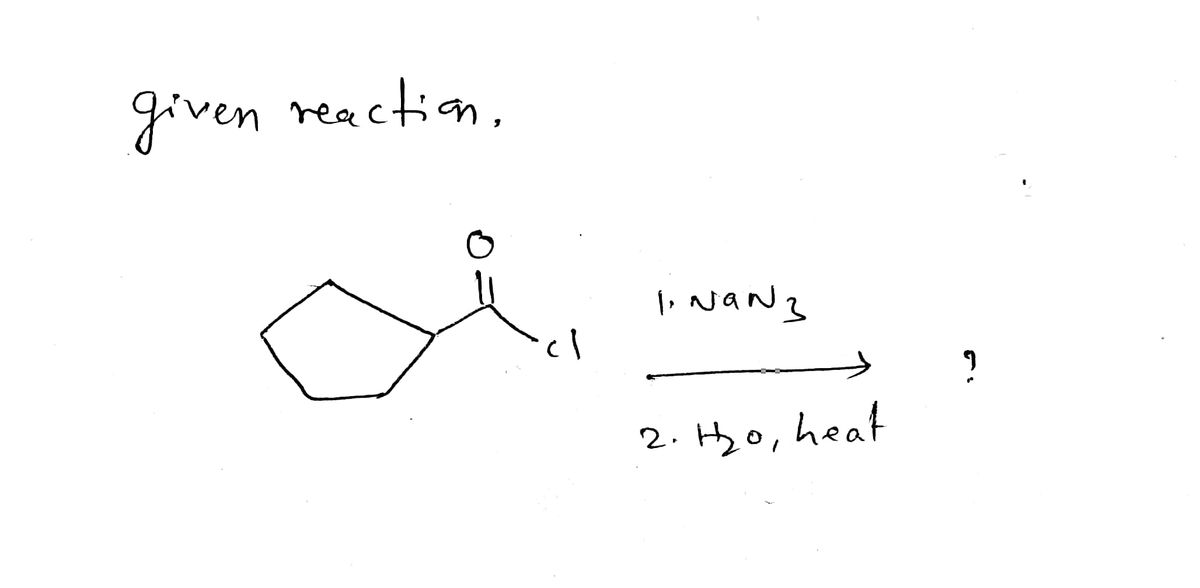
Solved) - A) op beste (CH3)2NH pH = 4.5 B) a Ime 2) NH2NH2, heat 1) NaN3 2)... (1 Answer) | Transtutors

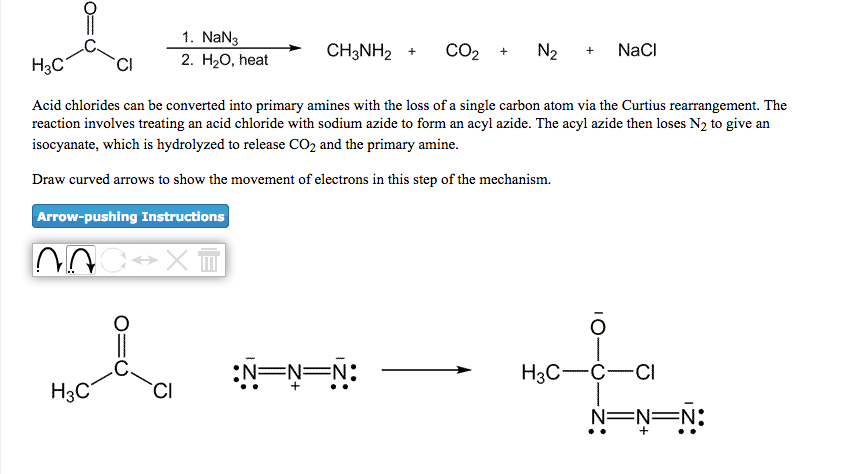
NaN3 → Product: H2O -NH2 only (b) (O)-NH, and HCOOH ca nu, only o O ENH: cod_OH + NH, NH2 (C) C-OH + NH3

Decade Advances of NaN3 in Three‐component Reactions - Wang - 2023 - Asian Journal of Organic Chemistry - Wiley Online Library
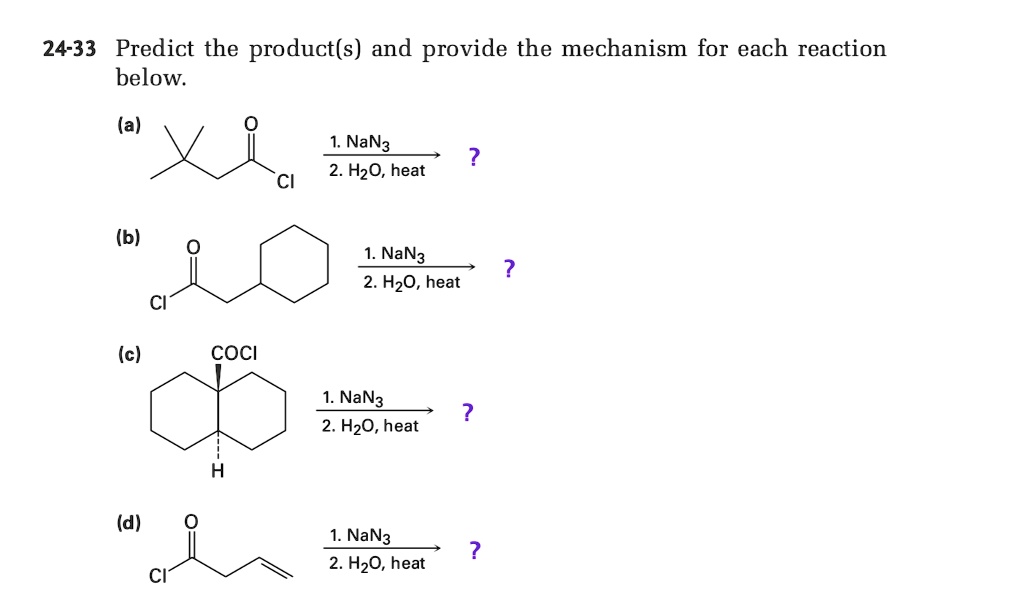
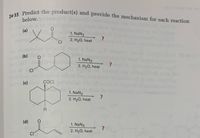
SOLVED: 24-33 Predict the product(s) and provide the mechanism for each reaction below: (a) NaN3 + H2O, heat (b) NaN3 + 2H2O, heat (c) COCl2 + NaN3 + 2H2O, heat (d) NaN3 + 2H2O, heat

Curtius rearrangement RCOCI (i) NaN3/C,H,OH, A (ii) H2O* or NaOH, A Schmidt reaction N3H/H2SO4(conc.) RCOOH^3

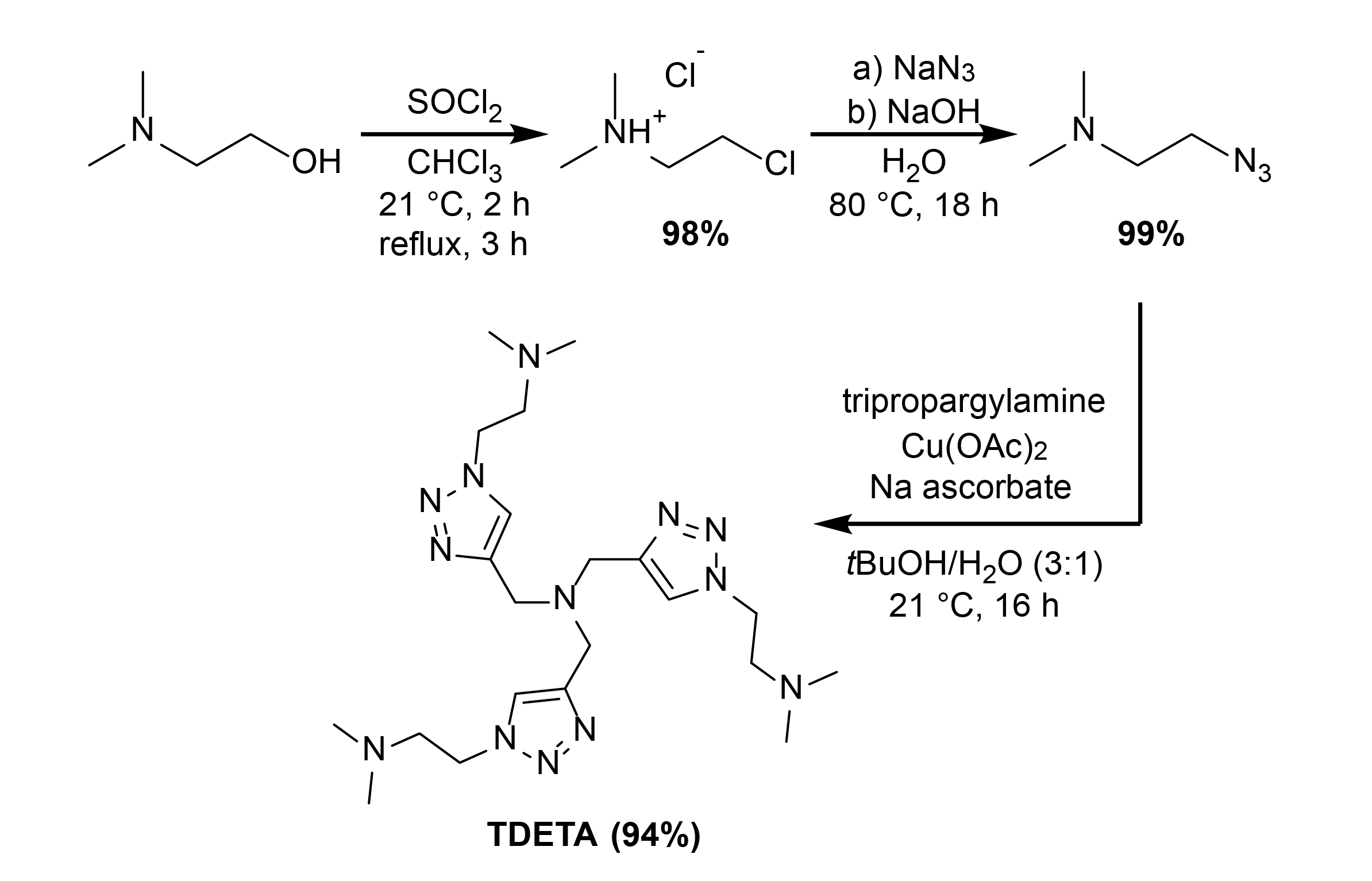
Copper-Catalyzed Azide–Alkyne Cycloaddition of Hydrazoic Acid Formed In Situ from Sodium Azide Affords 4-Monosubstituted-1,2,3-Triazoles | The Journal of Organic Chemistry