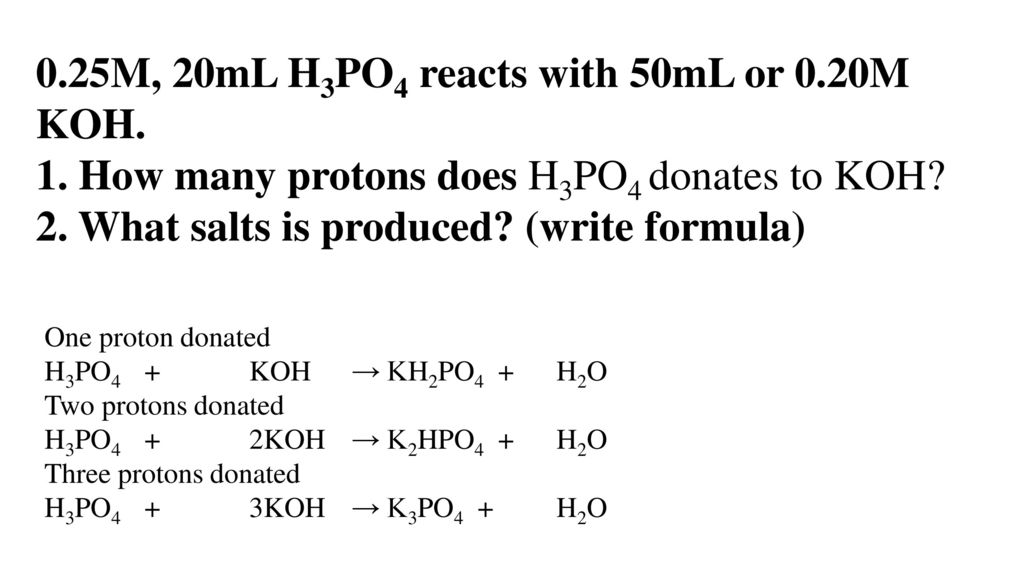
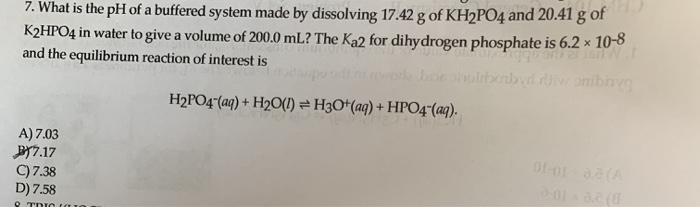KH2PO4 crystallisation from potassium chloride and ammonium dihydrogen phosphate – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.
Comment calculer le pH d'une solution de KHCO3 (2M) et acide citrique (1M) (1:1) en présence d'un tampon phosphate (PBS : 10mM Na2HPO4 et KH2PO4 1,8 mM) ? - Quora

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

Equilibrium phase diagram of the ternary system KH2PO4 + KNO3 + H2O at... | Download Scientific Diagram

Equilibrium phase diagram of the ternary system KH2PO4 + KNO3 + H2O at... | Download Scientific Diagram

SOLVED: A 0.492-g sample of KH2PO4 is titrated with 0.112 M NaOH, requiring 25.6 mL: H2PO4- + OH- → HPO42- + H2O What is the percent purity of the KH2PO4 (FW = 136.09)?

OneClass: Spectrophotometric analysis of phosphate can be performed by the following procedure: A. KH...
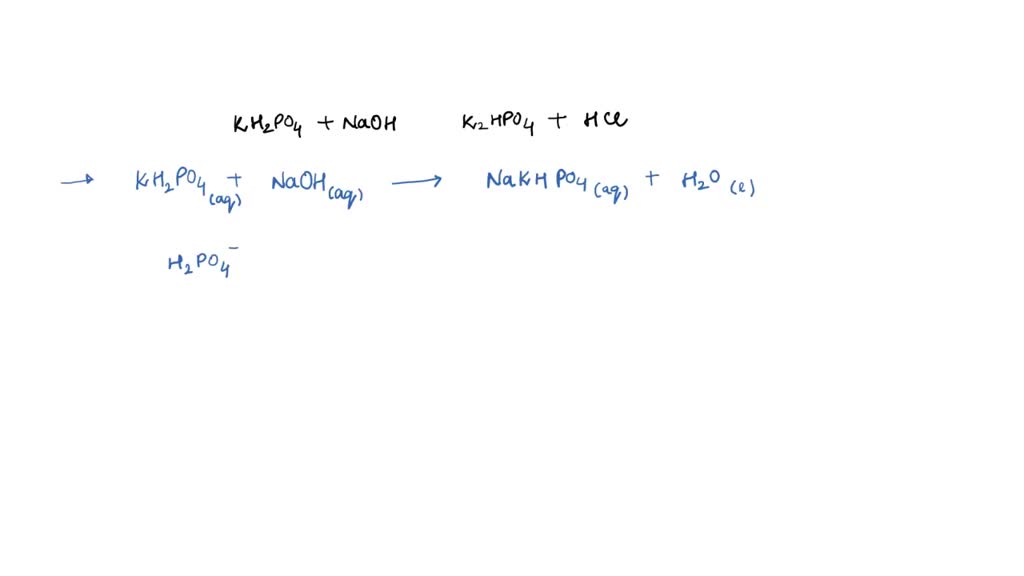
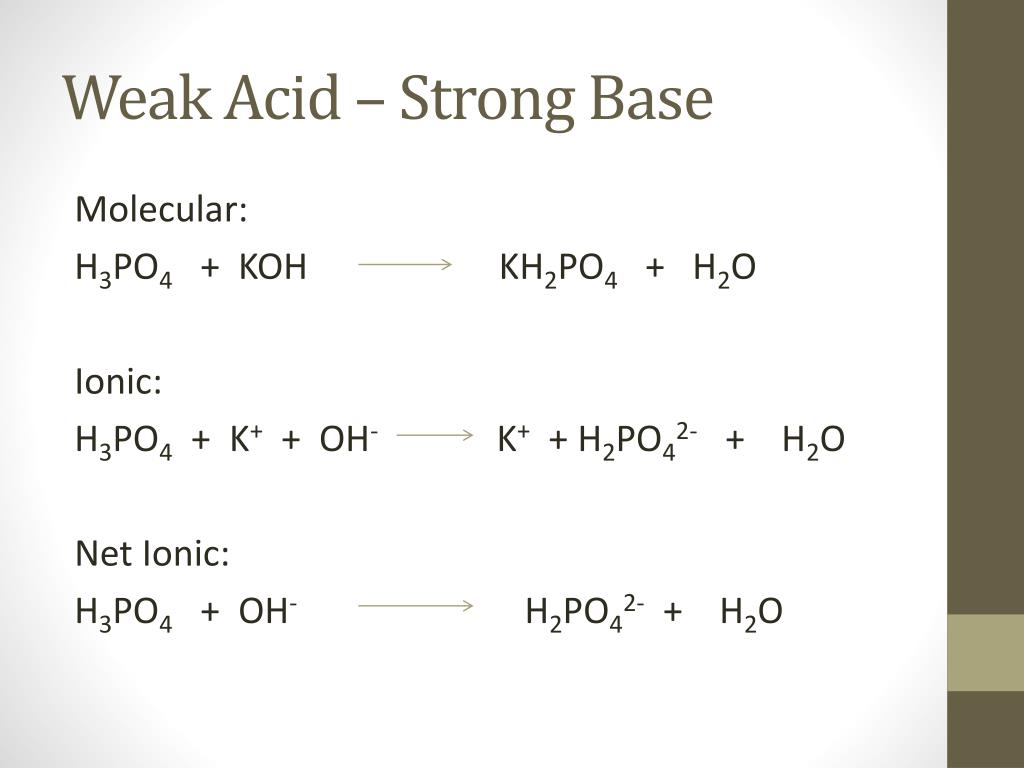
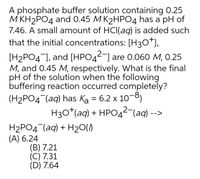
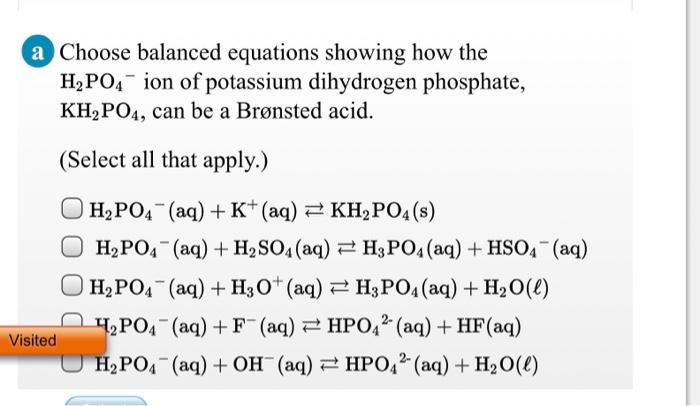
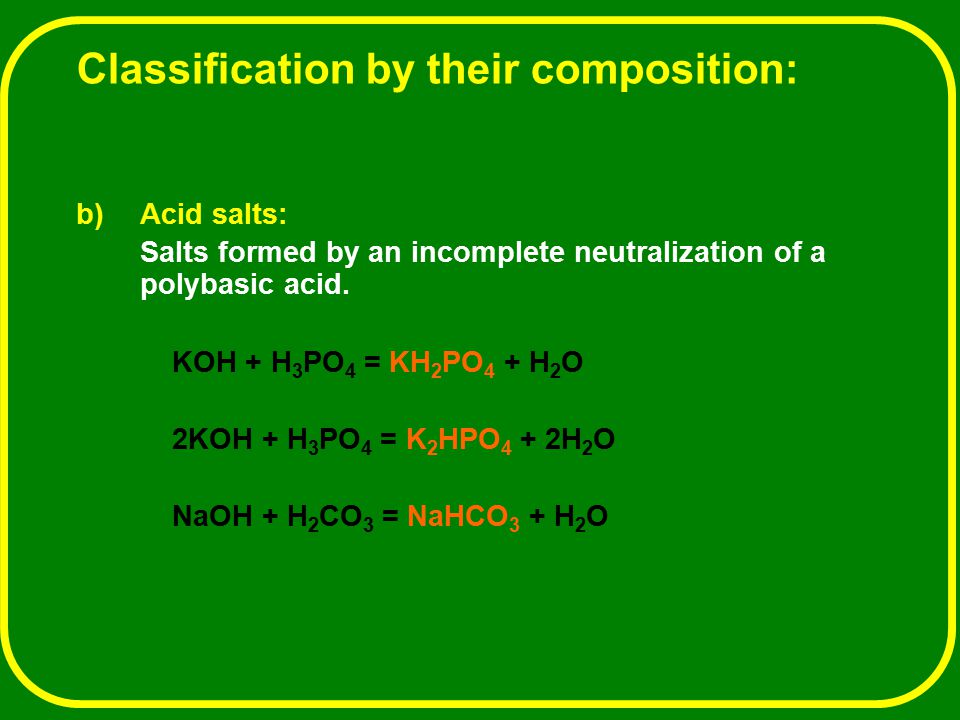
SOLVED: Write the equation and the reaction of the buffer solution KH2PO4 /K2HPO4 when NaOH and HCl is added
![FilSciHub Ed - CHEMISTRY MODULE] CHEMICAL REACTIONS & CHEMICAL EQUATIONS [ANSWER KEY] — Filipino Science Hub FilSciHub Ed - CHEMISTRY MODULE] CHEMICAL REACTIONS & CHEMICAL EQUATIONS [ANSWER KEY] — Filipino Science Hub](https://images.squarespace-cdn.com/content/v1/5f02d28f35d64d2a5022eeb1/1611724367429-9L5VAR6883R3CB69ME9Q/20.png)
FilSciHub Ed - CHEMISTRY MODULE] CHEMICAL REACTIONS & CHEMICAL EQUATIONS [ANSWER KEY] — Filipino Science Hub