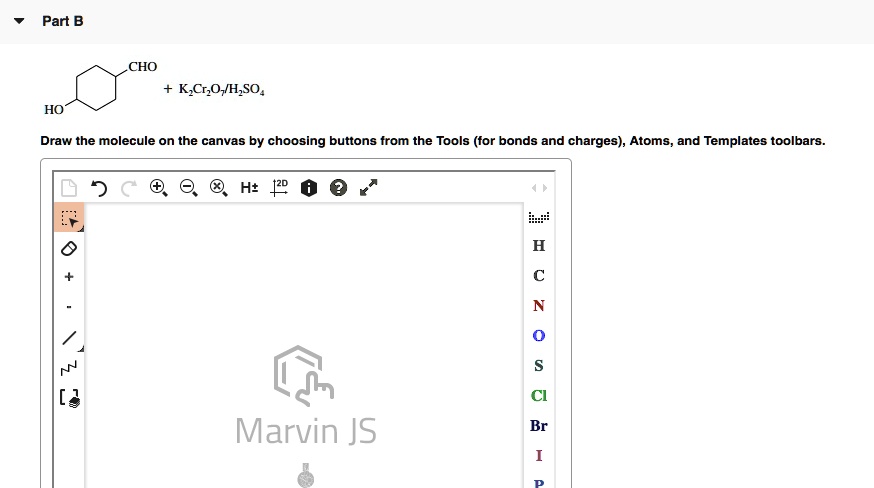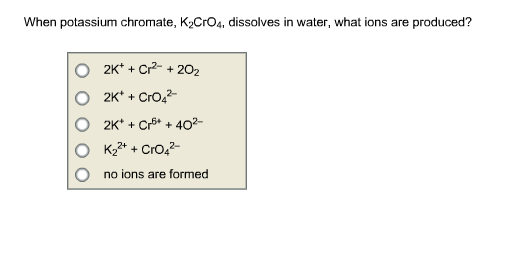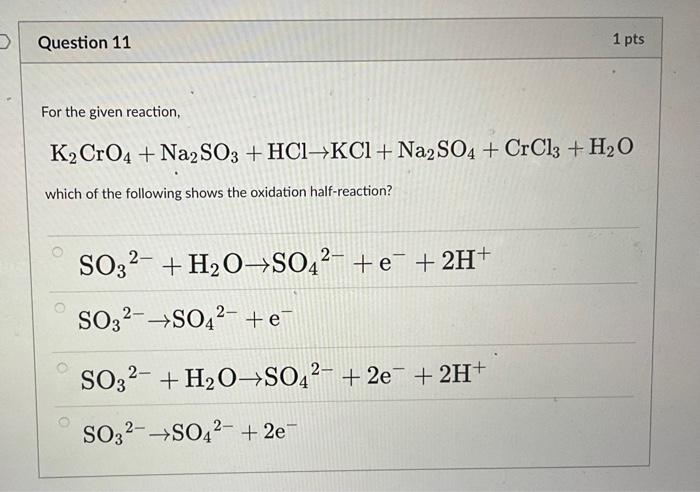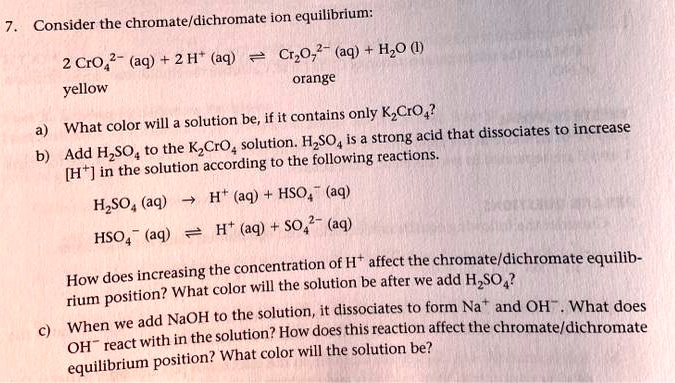
SOLVED: Consider the chromate/dichromate ion equilibrium: CrO4^2- (aq) + H2O (l) ⇌ 2 CrO4^2- (aq) + 2 H+ (aq). The solution will be orange-yellow if it contains only K2CrO4. What color will

A student titrates a 50.0 mL sample of chromic acid, H2CrO4, with 0.200 M KOH (aq). The titration reaches - Brainly.in

K2CrO4 + K2SO3 + H20 -> Cr(OH)3 + K2SO4+ KOH Uzupełnij współczynniki Bilansem jonowo-elektronowym. - Brainly.pl

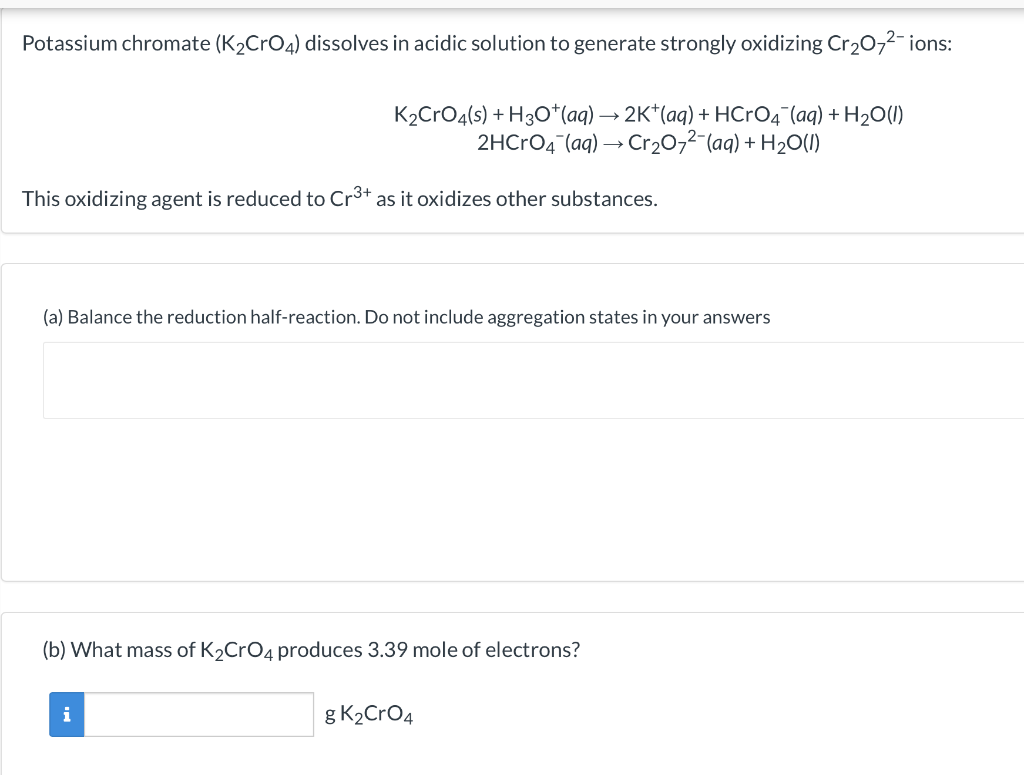
SOLVED: Potassium chromate (K2CrO4) dissolves in acidic solution to generate strongly oxidizing CrO42- ions: K2CrO4(s) + H3O+(aq) â†' 2K+(aq) + HCrO4-(aq) + H2O(l) 2HCrO4-(aq) + CrO42-(aq) + H2O(l) â†' 3H+(aq) + 2Cr3+(aq) +
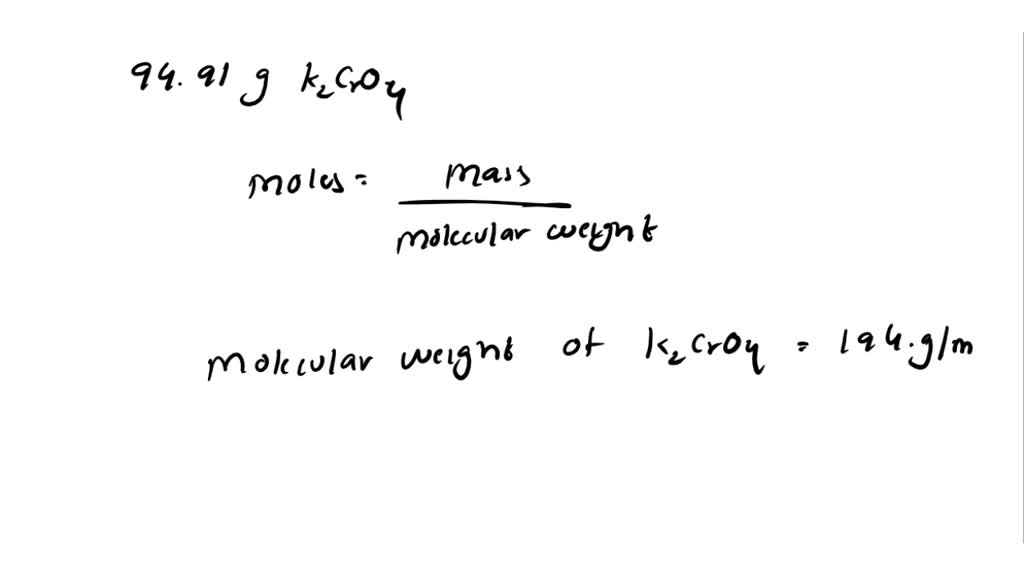
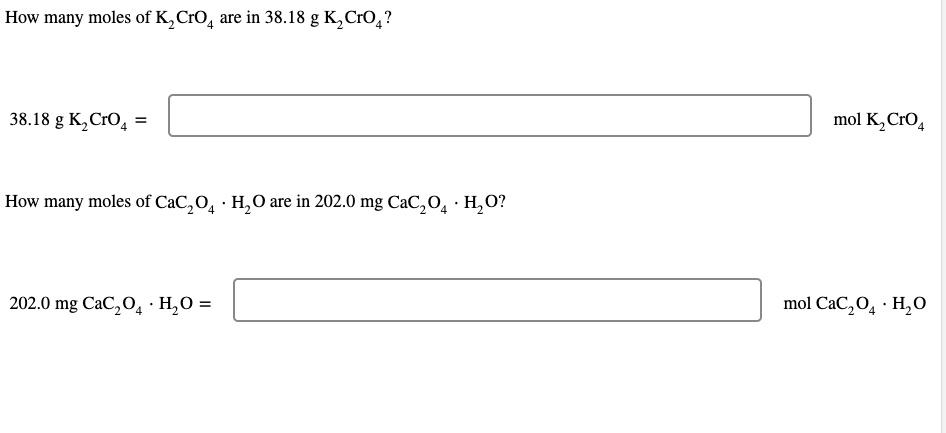
SOLVED: A. How many moles of K2CrO4 are in 94.91 g K2CrO4? B. How many moles of CaC2O4·H2O are in 236.0 mg CaC2O4·H2O?
Q117) The set of numerical coefficients that balances the equation K2CrO4 + HCl → K2Cr2O7 + kCl + H2O is kerala CEE 2001 d) 2, 2, 1, 2, 1
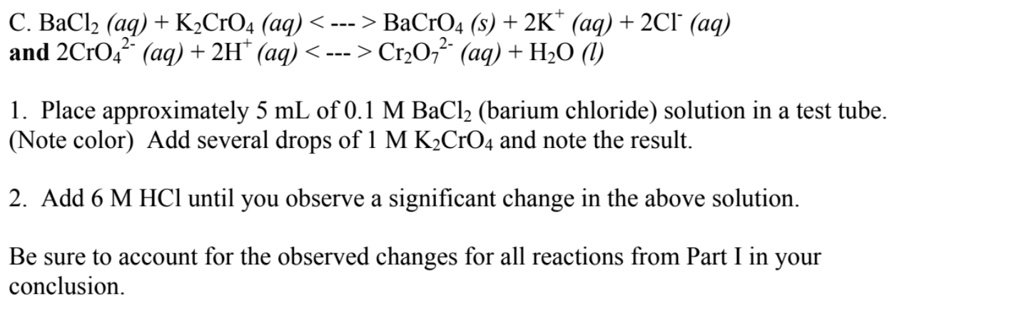
SOLVED: C.BaCl2 (aq) + K2CrO4 (aq) -> BaCrO4 + 2KCl (aq) 2CrO4^2- (aq) + 2H+ (aq) -> Cr2O7^2- (aq) + H2O Place approximately 5 mL of 0.1 M BaCl2 (barium chloride) solution

The of numerical coefficient that balance the equation K2CrO4 + HCI – K2Cr2O7 + KCI + H20 K2CrO4 + HCI - K2Cr20+ KCI + H20 31fAT HIMA BI Hahalg, ufa g A. 1, 1, 2, 2,1 B. 2, 2, 1, 1, 1 C. 2, 1, 1, 2,1 D. 2, 2, 1, 2,1