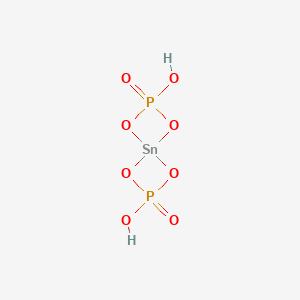
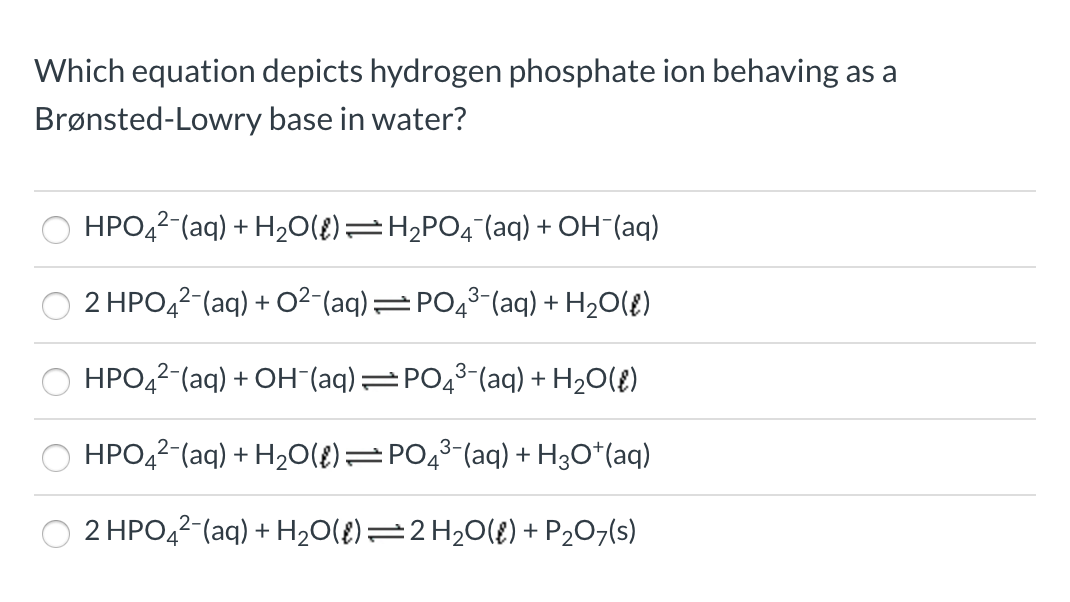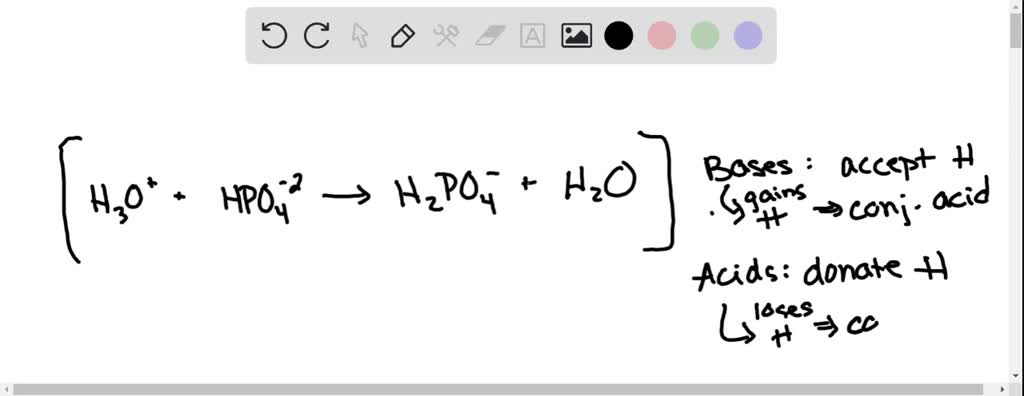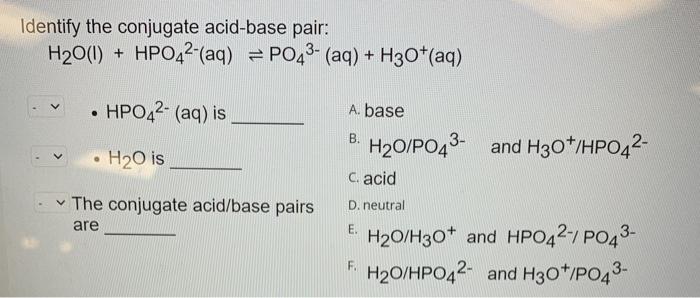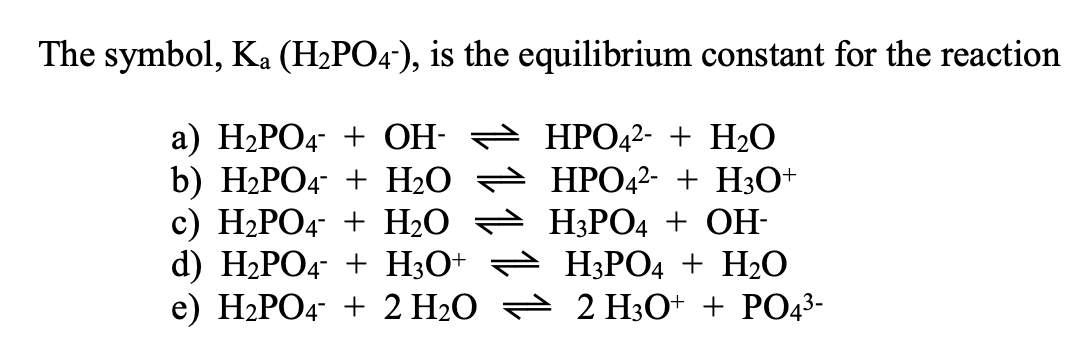Nucleation & growth of α-Ti(HPO4)2·H2O single-crystal and its structure determination from X-ray single–crystal data - ScienceDirect
CasNo.13772-29-7,Zirconium Phosphate Molecular Formula Zr(HPO4)2·H2O Change The Sewage Become Cleaning Water,(13772-29-7) Suppliers

Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol Using Ag@α-Ti(HPO4)2· H2O: Experimental and Computational Studies | Industrial & Engineering Chemistry Research

PDF) Neutron powder diffraction study of α-Ti(HPO4)2.H2O and α-Hf(HPO4)2.H2O; H-atom positions | Pilar Pertierra - Academia.edu
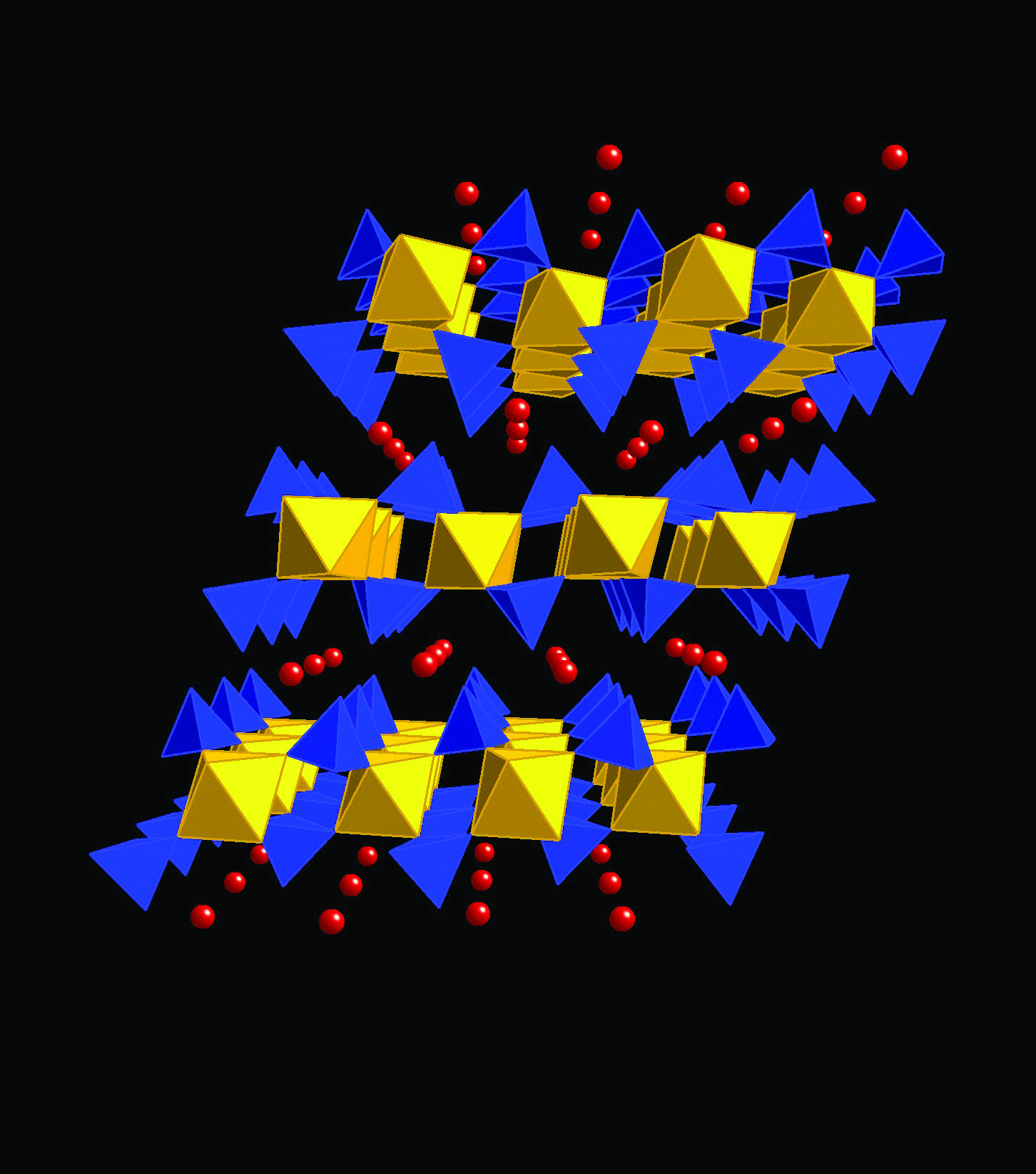
High resolution powder diffraction studies of mixed-metal layered phosphates - - Diamond Light Source

Figure 6 from VIIIVIV(HPO4)4·enH·H2O: a mixed-valence vanadium phosphate with an open framework | Semantic Scholar
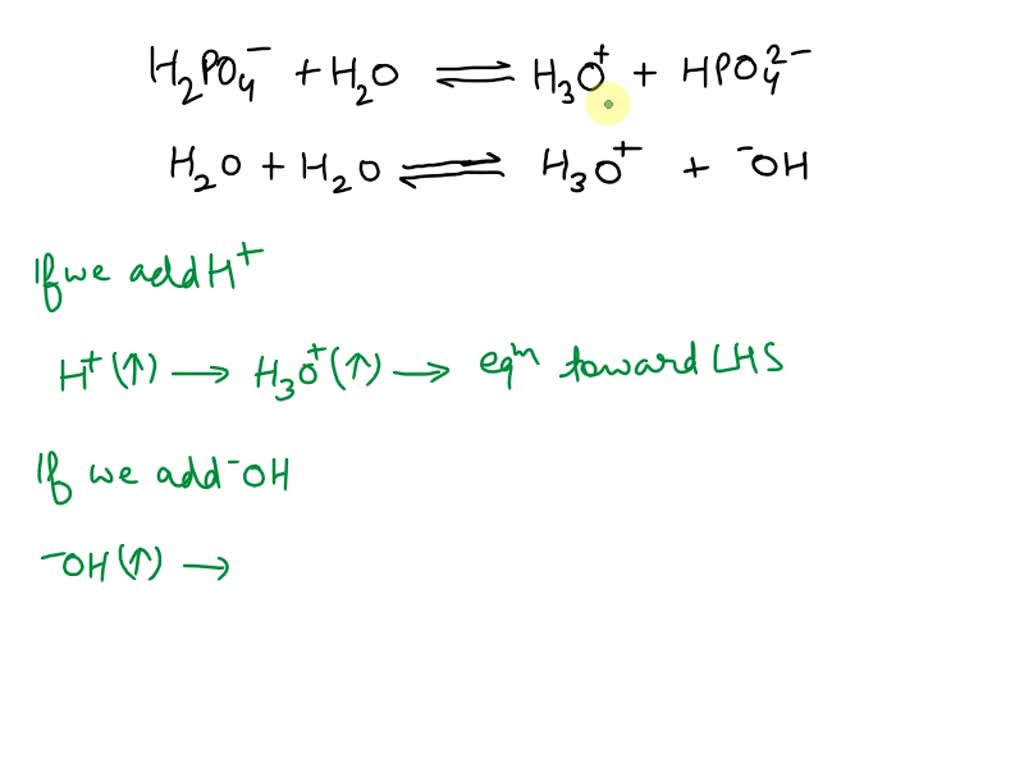
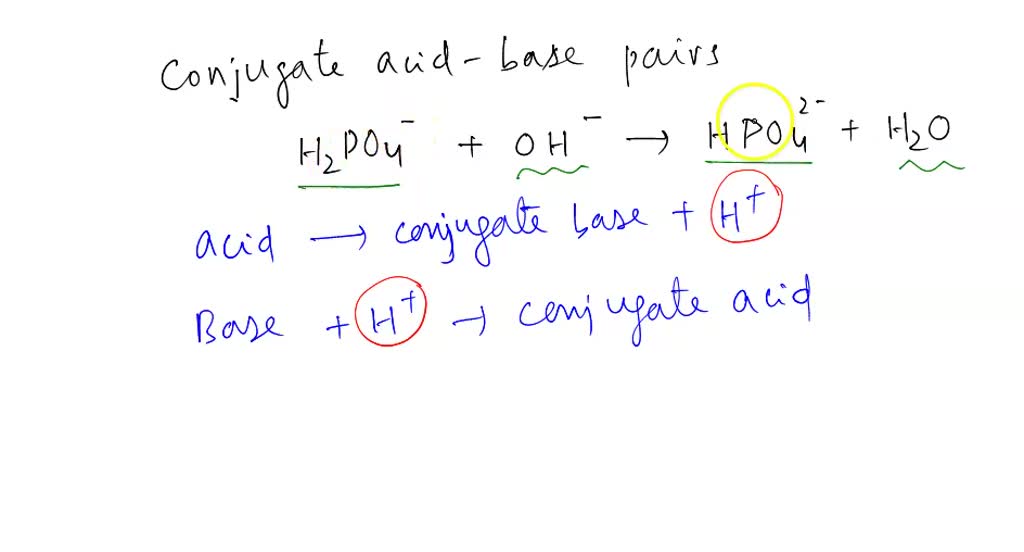
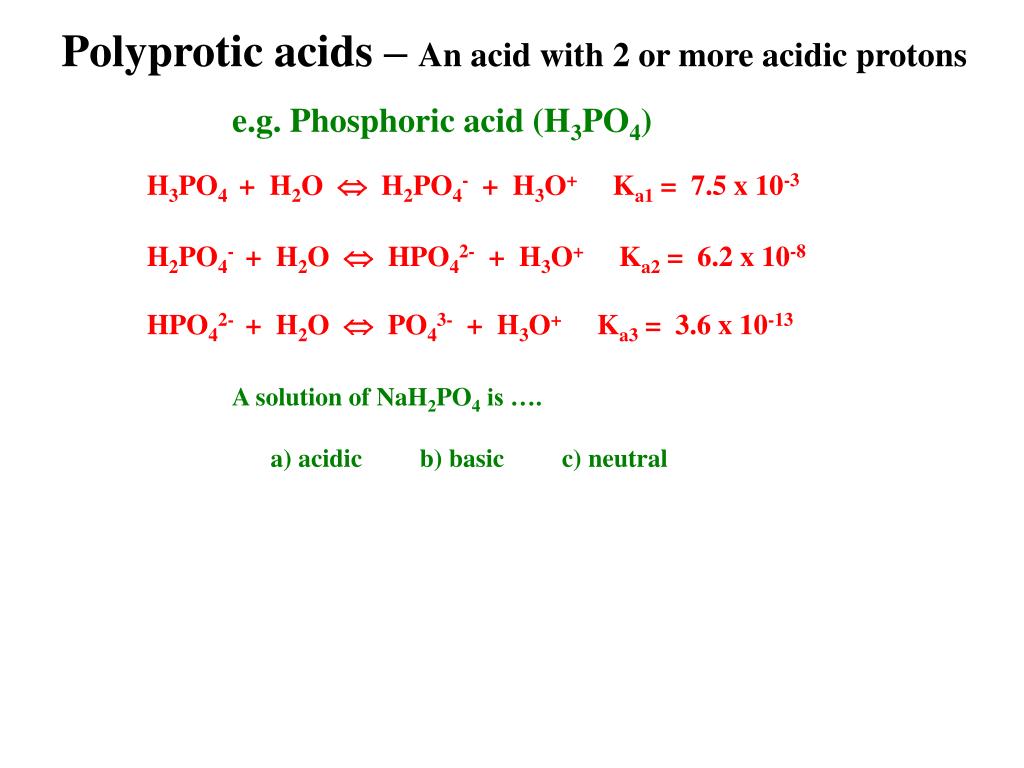
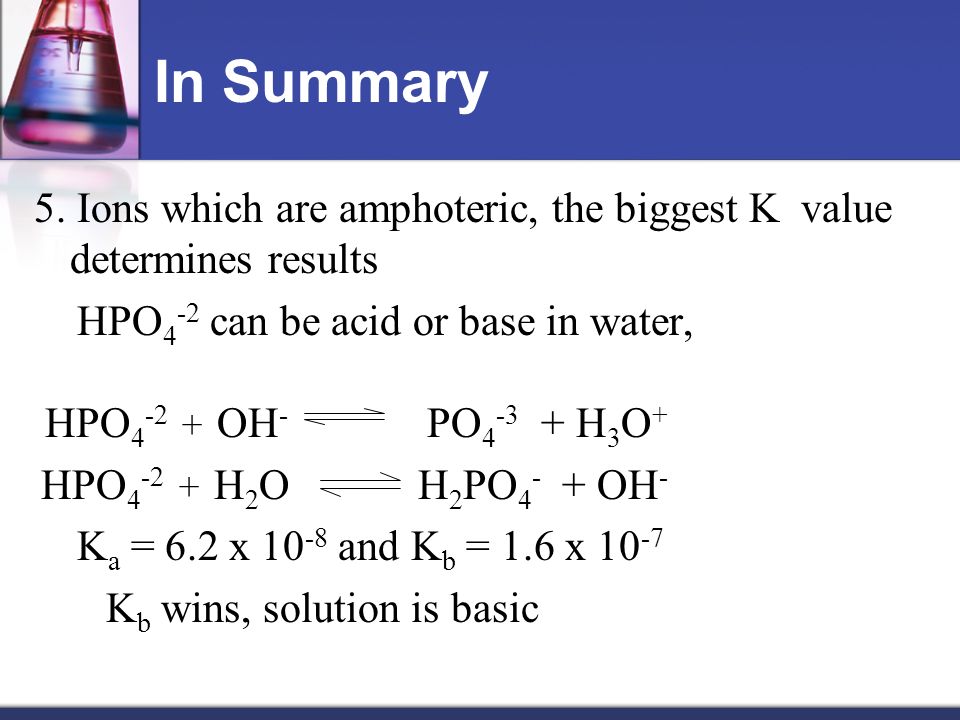
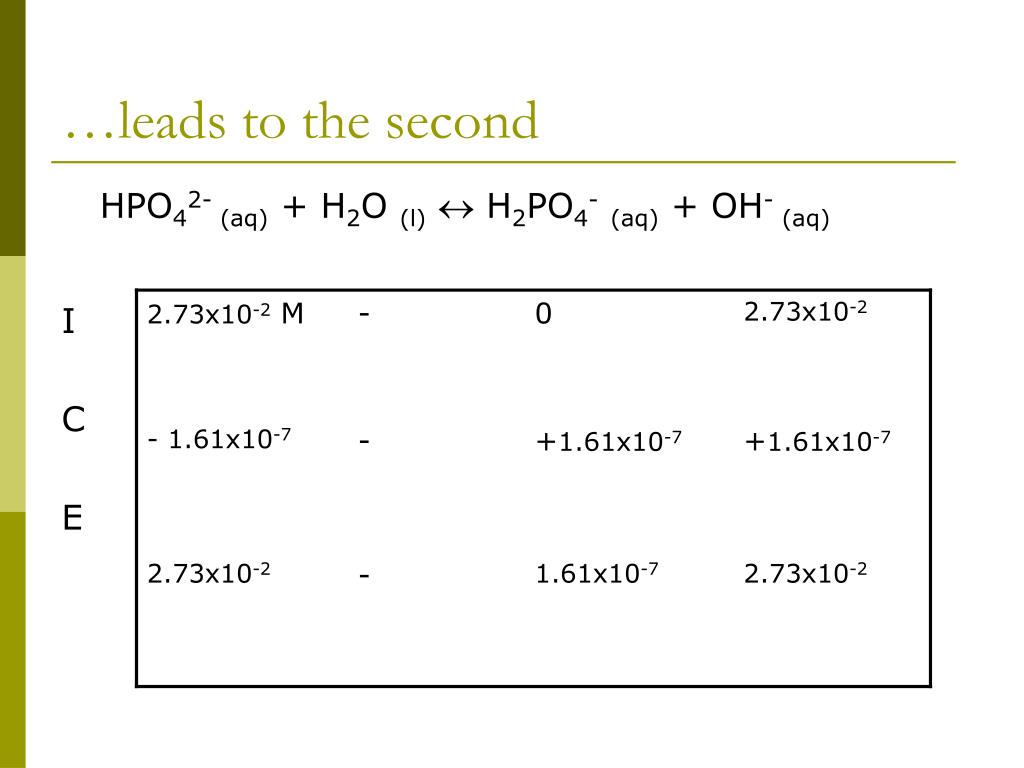
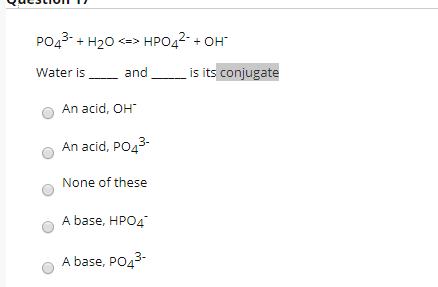
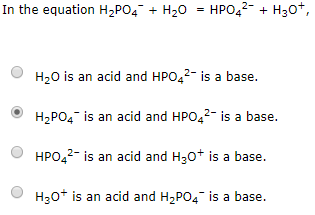
SOLVED: Relevant Equation H2PO4- + H2O <========> H3O+ + HPO4-2 Using Le Chatelier's Principle, explain why the phosphate buffer should have gone through a smaller change pH change compared to distilled water.

Single-crystal neutron diffraction and Mössbauer spectroscopic study of hureaulite, (Mn,Fe)5(PO4)2 (HPO4)2 (H2O)4 - European Journal of Mineralogy Volume 28 Number 1 — Schweizerbart science publishers
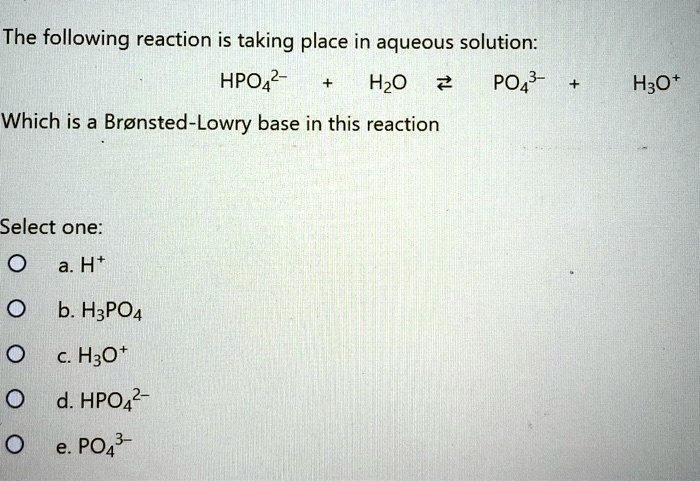
SOLVED: The following reaction is taking place in aqueous solution: HPO42- + H2O -> H3O+ + PO43-. Which is a Bronsted-Lowry base in this reaction? Select one: a. H+ b. H2PO4- c.

Catalytic conversions of isocyanate to urea and glucose to levulinate esters over mesoporous α-Ti(HPO4)2·H2O in green media - X-MOL

Figure 5. XRD of Zr0.8Ti0.2(HPO4)2.H2O : α- Zirconium Titanium Phosphates - Fibrous Cerium Phosphate Composite Membranes and Their 1,10- Phenanthroline Cu(II) Pillared Materials : Science and Education Publishing
![Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.1c02685/asset/images/large/ic1c02685_0008.jpeg)
Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry