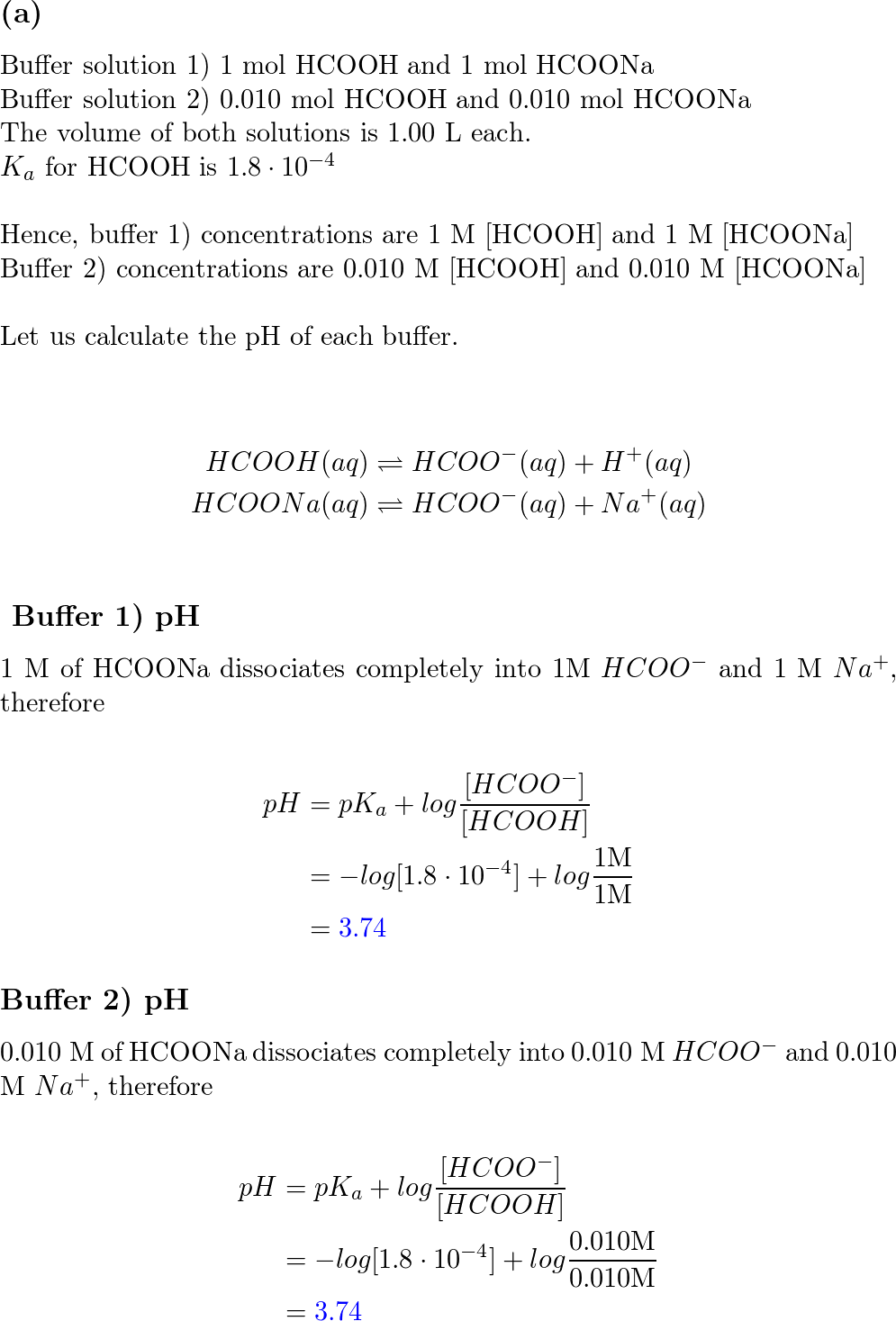
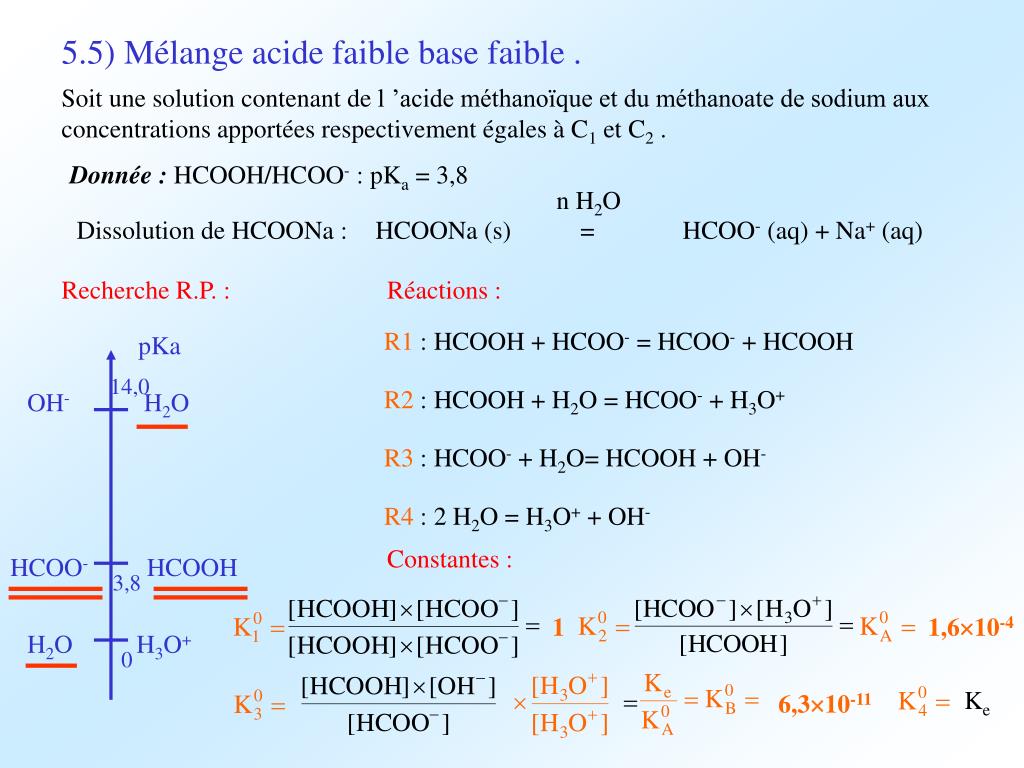
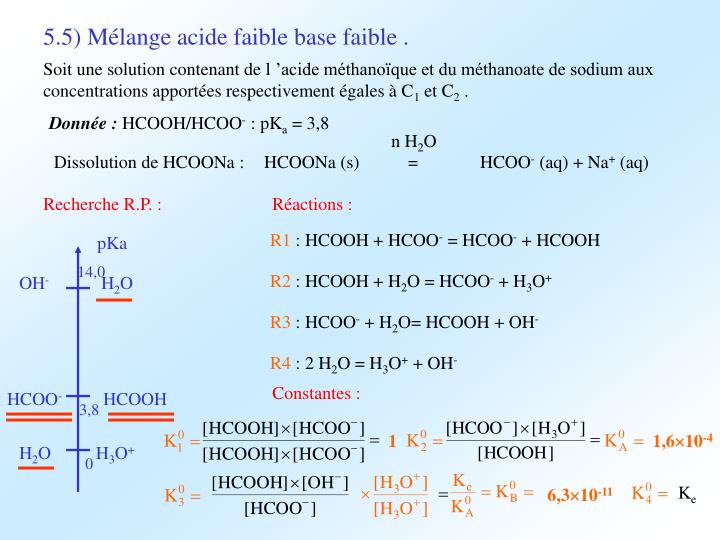
Find the equilibrium constant equilibrium HCOO + H2O + HCOOH + OH- In a solution of 0.1 M HCOONa. K (HCOOH) = 1.8 x 104 (1) 1.8 x 10-4 (275.56 10 (3) 5.56 x 10-11 (4) 1.8 x 10-18

SOLVED: A 1.0 L buffer solution is prepared with 0.25 M formic acid (HCOOH) and 0.50 M sodium formate (HCOONa) - HCOOH(aq) + H2O(aq) = HCOO-(aq) + H3O+(aq). pKa = 3.74. What

Find the equilibrium constant equilibrium HCOO + H2O HCOOH + OH- In a solution of 0.1 M HCOONa. Ka(HCOOH) = 1.8 * 104 (1) 1.8 x 10-4 (2) 5.56 x 10° (4) 1.8 * 10-18 (3) 5.56 x 107-11

Experimental phase equilibrium data for the NPG (1) + HCOONa (2) +H2O... | Download Scientific Diagram
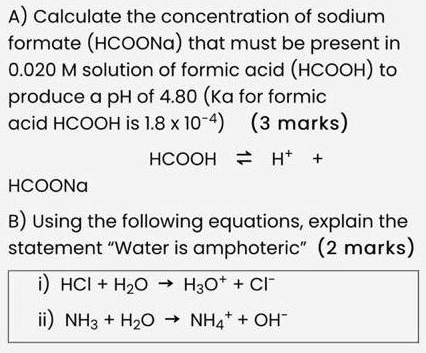
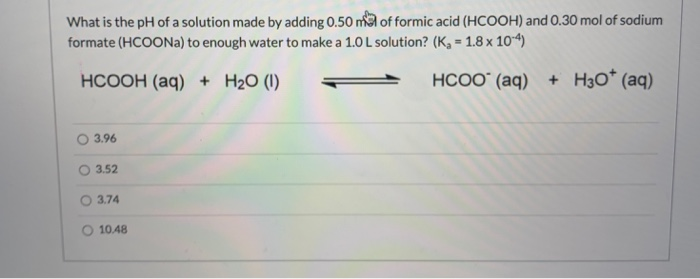
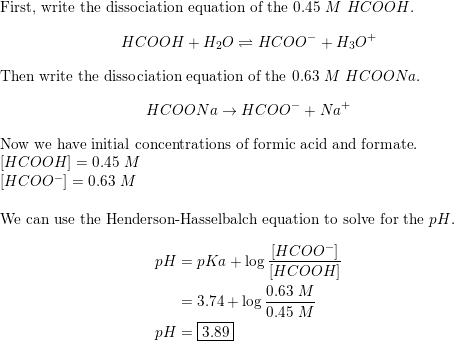
SOLVED: A) Calculate the concentration of sodium formate (HCOONa) that must be present in a 0.020 M solution of formic acid (HCOOH) to produce a pH of 4.80 (Ka for formic acid

Solved) - Which equation is correct for a buffer solution of formic acid and... (1 Answer) | Transtutors
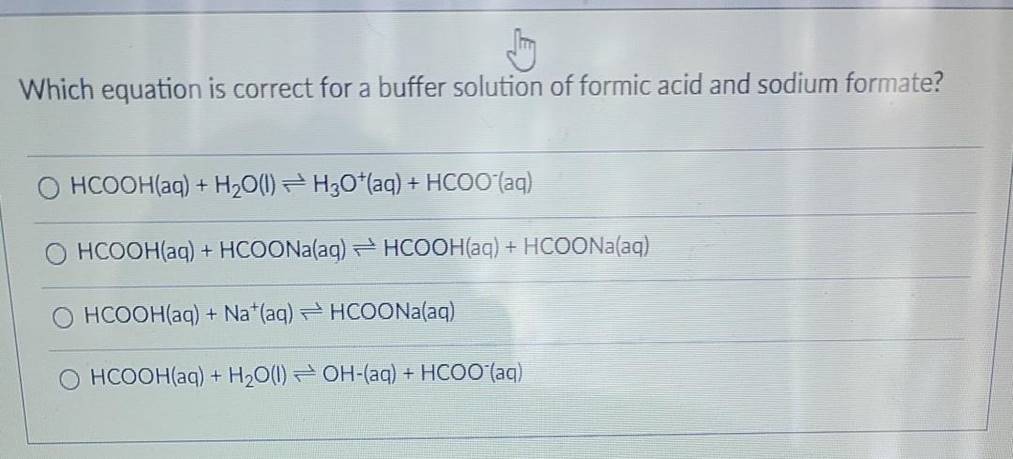
Solved) - Which equation is correct for a buffer solution of formic acid and... (1 Answer) | Transtutors

Find the equilibrium constant equilibrium HCOO + H2O HCOOH + OH- In a solution of 0.1 M HCOONa. Ka(HCOOH) = 1.8 * 104 (1) 1.8 x 10-4 (2) 5.56 x 10° (4) 1.8 * 10-18 (3) 5.56 x 107-11