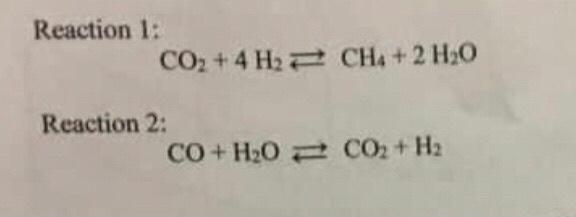For the reaction: Co(H2O)6^{2+} + 4Cl^- \leftrightharpoons CoCl4^{2-} + 6H2O After adding 12M HCl, what would be the roles of H^+ ions and Cl^- ions? | Homework.Study.com

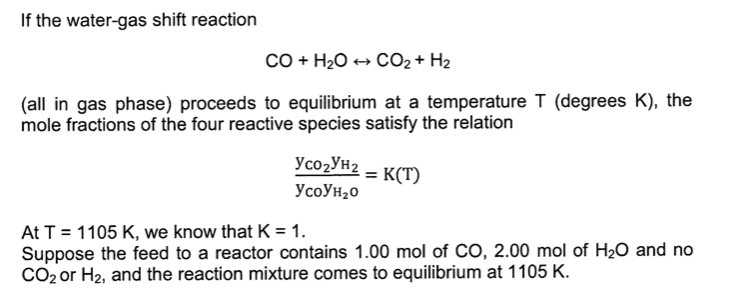
64 The equilibrium constant the reaction, CO(g) + H2O (9) CO2 (g) + H2 (g) a certain temperature is 2.2. Initially one mole of CO and one mole of H2O are placed

Reaction of CO, H2O, H2 and CO2 on the clean as well as O, OH and H precovered Fe(100) and Fe(111) surfaces - Catalysis Science & Technology (RSC Publishing)

Radiation-induced synthesis of formic acid in the H2O–CO system: A matrix isolation study - ScienceDirect
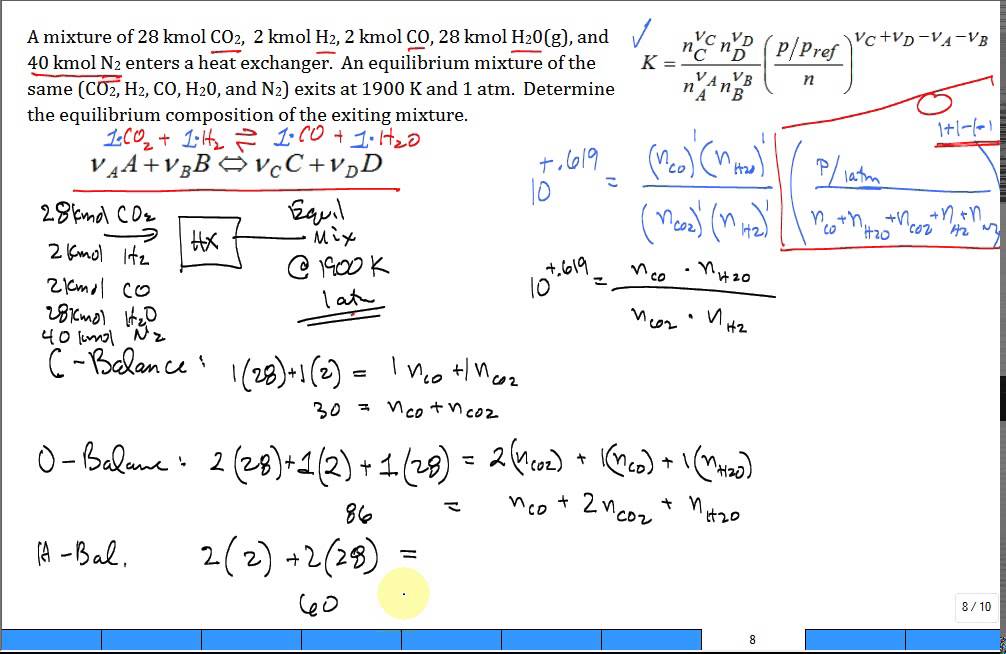
Kp for the reaction CO2 + H2 =CO + H2O is found to be 16 at a given temperature. Originally equal number of moles of H2 and CO2 were placed in the
CO2+H2=CO+H2O, 1 mole of CO2 and 2 moles of H2 are placed in a 2L container. At equilibrium, the concentration of CO is 0.28 mol/L. What is the equilibrium constant Kc for

CO+H2O=CO2+H2 balance the chemical equation by law of conservation of mass @mydocumentary838. - YouTube
![Consider this reaction: [Co(H2O)6]2+ + 4 Cl- -> ? [CoCl4]2- + 6 H2O The octahedral starting material is pink in color, absorbing visible light with ?max = 510 nm. The tetrahedral product Consider this reaction: [Co(H2O)6]2+ + 4 Cl- -> ? [CoCl4]2- + 6 H2O The octahedral starting material is pink in color, absorbing visible light with ?max = 510 nm. The tetrahedral product](https://homework.study.com/cimages/multimages/16/studycomoctahedraltetrahedraldq10shownd7system1969898108720194826.png)
Consider this reaction: [Co(H2O)6]2+ + 4 Cl- -> ? [CoCl4]2- + 6 H2O The octahedral starting material is pink in color, absorbing visible light with ?max = 510 nm. The tetrahedral product


![co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why](https://cdn.eduncle.com/library/scoop-files/2021/9/can_image_1631894248696.jpg)
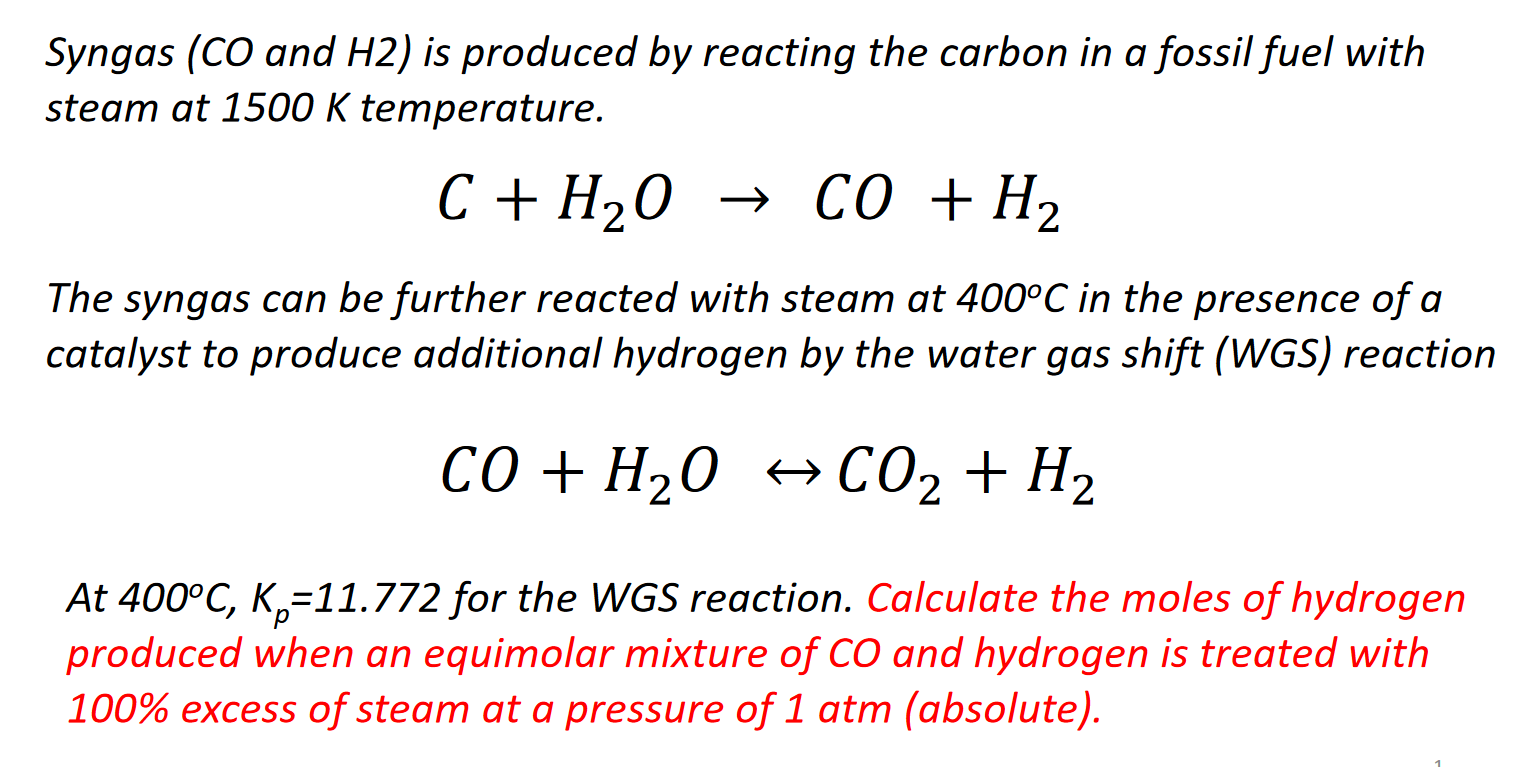

![Co(H2O)6]2+ Co(H2O)6]2+](https://tp-inorga-1-13.webself.net/file/si532904/CoH2O6-fi8185737x250.png)