Self-Supplying of Hydrogen Peroxide/Oxygen Based on CaO2-Co3O4 Cascade Nanoreactors for Cellular Microenvironment Regulation | ACS Applied Nano Materials
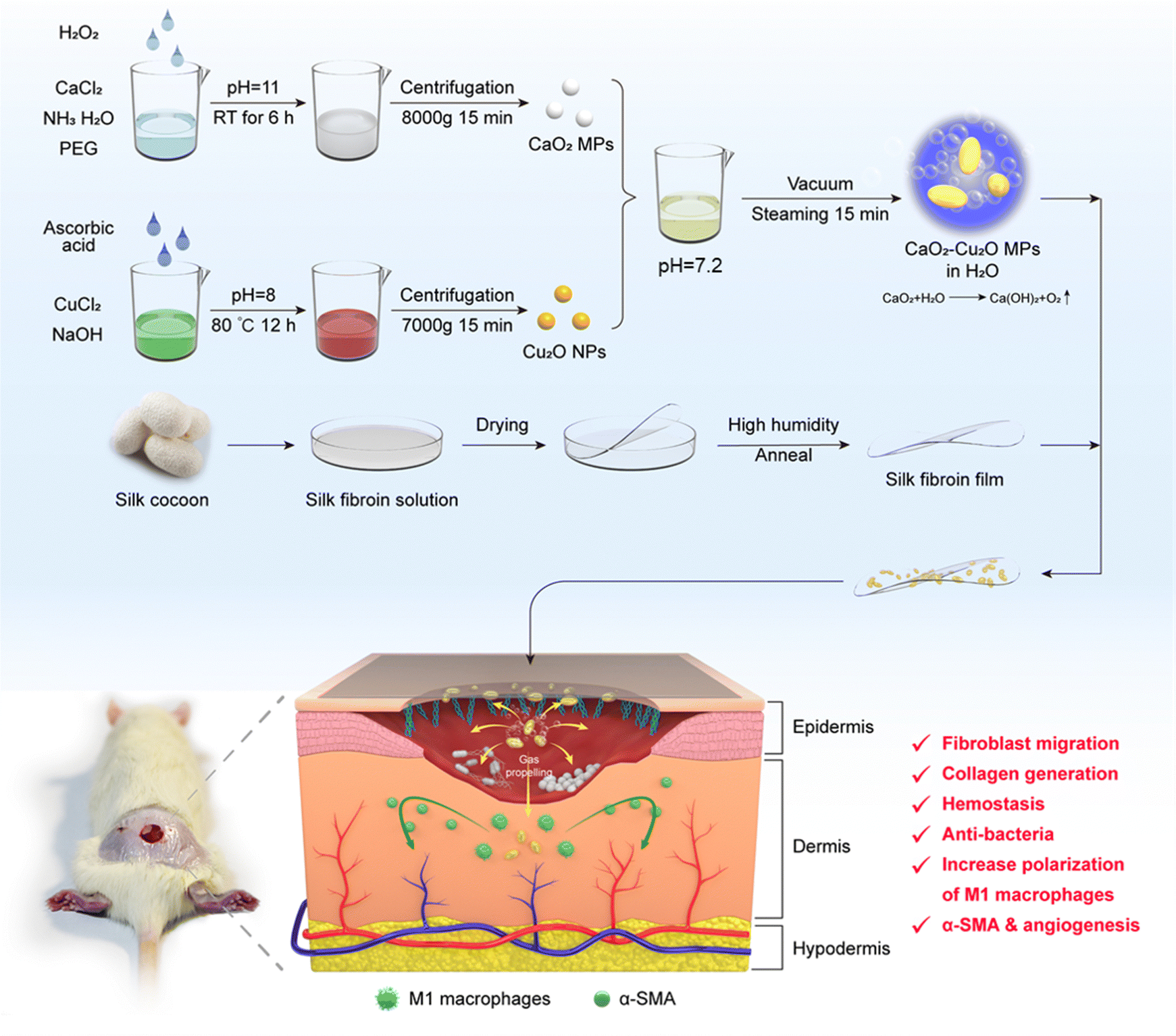
CaO 2 –Cu 2 O micromotors accelerate infected wound healing through antibacterial functions, hemostasis, improved cell migration, and inflammatory reg ... - Journal of Materials Chemistry B (RSC Publishing) DOI:10.1039/D3TB02335D

Fabricating collagen films with oxygen-release capabilities: 1,7-octadiene PECVD encapsulation of calcium peroxide - American Chemical Society
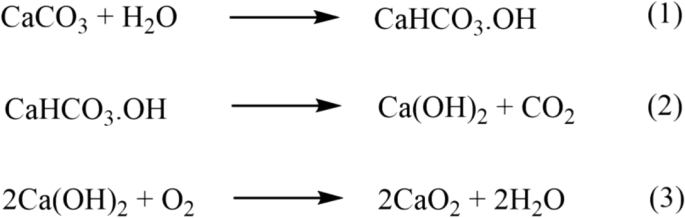
Novel iron sand-derived α-Fe2O3/CaO2 bifunctional catalyst for waste cooking oil-based biodiesel production | Environmental Science and Pollution Research

Identify the substance oxidized, substance reduced, oxidizing agent and reducing agent in the following: 1 Cl2 + 2NaBr 2NaCl + - Science - Chemical Reactions and Equations - 13638355 | Meritnation.com

SOLVED: Consider the combustion of Ca. What are the expected products of the reaction? Select all that apply: CaO2 CaZo H2O CaO CO2

Steady release-activation of hydrogen peroxide and molecular oxygen towards the removal of ciprofloxacin in the FeOCl/CaO2 system - ScienceDirect

Please help with all parts and show work. The decomposition of Ca(OH)_2(s) into CaO(s) and H_2O(g) at constant pressure requires the addition of 109 kj of heat per mole of Ca(OH)_2. (a)

C) Cal24 Pulice (d) All of these - Cl2 2 → A - Auto-oxidation Ca(OH)2 -H20 → CaCl2 + B Dry cao2 Identify B in the above reaction :