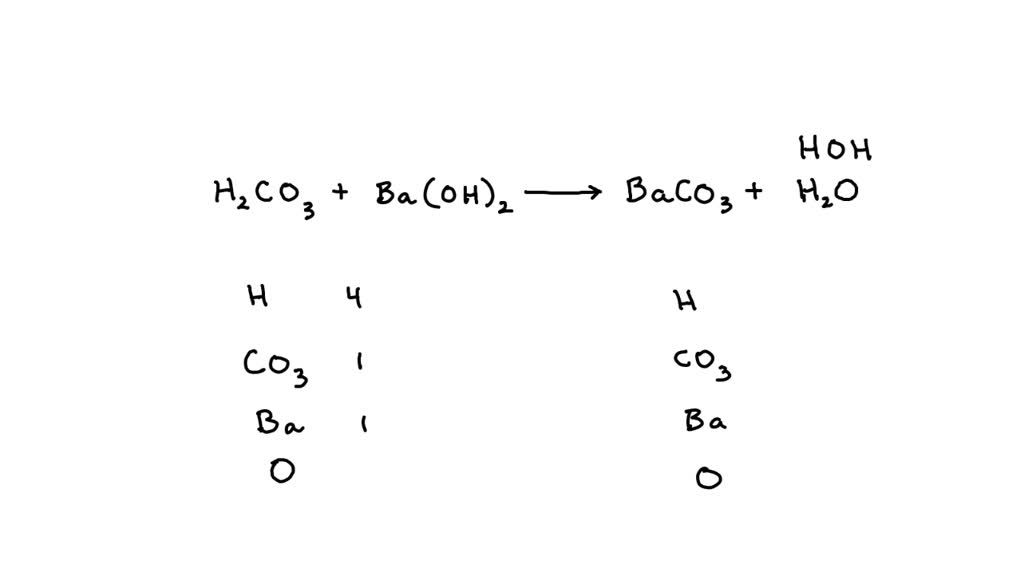Calculate the mass of BaCO3 produced when excess CO2 is bubbled through a solution containing 0.205 moles of Ba(OH)2. - Sarthaks eConnect | Largest Online Education Community

Cho sơ đồ các phản ứng theo đúng tỉ lệ mol:(a) X(t0) $ \to $ Y + CO2(b) Y + H2O $ \to $ Z(c) T + Z $ \to $ R + X + H2O(d?

The experimental enthalpies of solution of Y, BaCO3 and CoCl2·4.24H2O... | Download Scientific Diagram
Heritage | Free Full-Text | Characterization of Barium Hydroxide Used as Consolidating Agent for Monumental Surfaces in Venice
Trajectory and timescale of oxygen and clumped isotope equilibration in the dissolved carbonate system under normal and enzymati

Synthetic pathway of 1. a) NH2OH·HCl, BaCO3, Pd/C, N2H4·H2O, reflux in... | Download Scientific Diagram

24.Write equilibrium constant expression the following reac (i) BaCO3 (8) ---------- BaO(s) + CO2 (g).

Question Video: Determining the Products of the Neutralization Reaction of Barium Hydroxide Ba(OH)₂ with Carbonic Acid H₂CO₃ | Nagwa

a. SO2 + ? → H2SO3 b. ? +H2O → KOH c. ? + ?→ CaCO3 d. CO2 + ? → BaCO3 + H2O e. ? + H2S04 → MgSO4 + H2O f. Fe + HCl– → ? + ? g. Al203 + H2SO4 → ? + H2O h. N

PPT - What is the difference between a chemical reaction and physical change? PowerPoint Presentation - ID:5813241

Solve 53 sum 200 m' solution molarity of tho volumo ot CO, at S T P on heat'0 9 (2) - Chemistry - - 12898155 | Meritnation.com

SOLVED: Equation: Ba(OH)2(aq) CO2(g) BaCO3(s) H2O(l) Total ionic: Ba2+(aq) + 2OH-(aq) + CO2(g) â†' BaCO3(s) + H2O(l) Net ionic: Ba2+(aq) + CO2(g) â†' BaCO3(s) + H2O(l)
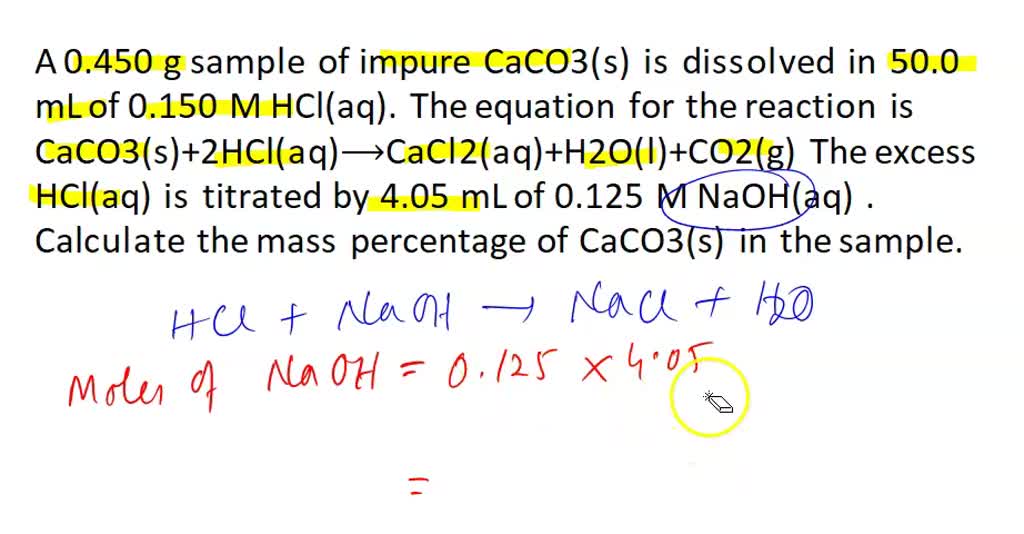
SOLVED: (b) The Ksp of barium carbonate, BaCO3, is 2.58×10^(-9). Calculate the molar solubility, S, of this compound. S= (c) A 0.450 g sample of impure CaCO3(s) is dissolved in 50.0 mL